Oral Abstract
Myocardial viability assessment prior to heart transplantation: a feasibility CMR study in a porcine model
- KB
Khaoula Bouazizi, PhD
Rsearch engineer
Laboratoire d'Imagerie Biomédicale, France - KB
Khaoula Bouazizi, PhD
Rsearch engineer
Laboratoire d'Imagerie Biomédicale, France - EB
Elodie Berg, MD
Doctor
Cardiovascular surgery department, Pitié-Salpêtrière Hospital, France - NL
Noémie Lacourt, BEng
Intern
Laboratoire d'Imagerie Biomédicale, France - MZ
Mohamed ZARAI, MSc
MSc
ICAN Imaging, Institute of Cardiometabolism and Nutrition (ICAN), Paris, France, France - FB
Francesca Branzoli, PhD
Researcher
Institut du Cerveau et de la Moelle épinière (ICM), France - ST
Solenn Toupin, PhD
PhD
Siemens Healthineers, France - YL
Yann Le Fur, PhD
Researcher
Center for Magnetic Resonance in Biology and Medicine (CRMBM), CNRS, Aix-Marseille Univ, France - MB
Monique Bernard, PhD
Physicist
Aix-Marseille Université, CNRS, CRMBM, Marseille, France - GL
Guillaume Lebreton, MD
MCU PH
Service de Chirurgie Thoracique et Cardiaque, Institut de Cardiologie, Groupe Hospitalier Pitié Salpêtrière APHP, Paris, France, France - AR
Alban Redheuil, MD, PhD
Professor
Unité d’Imagerie Cardiovasculaire et Thoracique (ICT), Pitié-Salpêtrière Hospital, France 
Nadjia Kachenoura, PhD
PhD
Laboratoire d'Imagerie Biomédicale, Sorbonne Université, France.jpg)
Frank Kober, PhD
Director of Research at CNRS
Aix-Marseille University, France
Presenting Author(s)
Primary Author(s)
Co-Author(s)
Improved myocardial viability evaluation is crucial to decrease the likelihood of early cardiac graft dysfunction1. CMR is the non-invasive reference method for myocardial structure and content characterization, including water/edema2, fibrosis3 and energy metabolism. Our aim was to establish an efficient protocol to assess myocardial viability over time. A fast method could help surgeons in evaluating graft quality prior to transplantation, enhancing the likelihood of transplantation success.
Methods:
Eight pigs were included in this study. After cardioplegia, the hearts were: 1) placed in a polystyrene container filled with cardioplegic solution, 2) fixed, 3) imaged using a whole-body 1.5T MRI system (MAGNETOM Sola, Siemens Healthineers, Germany) with an 18-element array surface coil.
1H-MRS spectra were obtained by PRESS with a voxel placed in the interventricular septum using the four-chamber and short-axis views with the following parameters: TE/TR(ms): 135/1500, 1024 data points, bandwidth:1000 Hz. After automated shimming, water-suppressed spectra were acquired to quantify intramyocardial metabolites. Of note, quantification of intramyocardial metabolites was performed using 32 to 512 NEX. Non-water-suppressed spectra were acquired with 4 to 16 NEX and used as a reference. Myocardial T1 mapping was obtained using a MOLLI sequence with the following imaging parameters: FoV: 150x175mm2, matrix: 110x128, slice thickness: 8mm, TE/TR(ms): 1.21/2.3. Spectroscopy and T1 mapping acquisitions were repeated hourly over a total period of 4 hours.
Spectra were fitted using a software as previously described4 and a positive peak at 1.3ppm corresponded to combined lactate and lipids signals while a peak at 3.0 ppm was related to creatine5. Intramyocardial metabolite contents relative to water were calculated and expressed as a percentage.
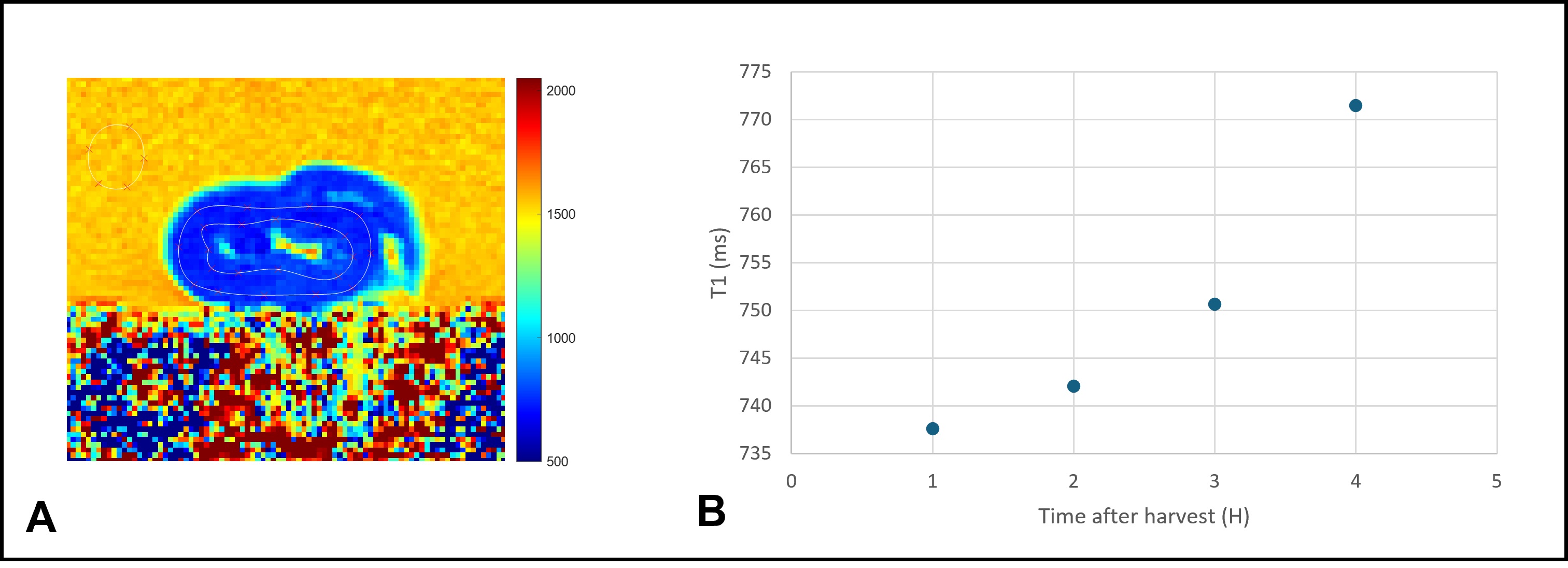
For mean T1 measurements, the midventricular short-axis MOLLI image was manually contoured to outline the endocardium and the epicardium and such contours were superimposed on all T1 maps acquired over time.
Results:
It was possible to quantify intramyocardial metabolites in 75% of hearts. The other spectra could not be used due to a low signal-to-noise ratio. Figure 1 shows a typical water suppressed 1H-MRS spectra obtained by PRESS one hour after heart explant.
The mean intramyocardial lipid and lactate concentrations were 0.024±0.012, 0.033±0.006, 0.033±0.01, 0.037±0.013%, at the first, second, third and fourth hour after heart explantation respectively. This indicates a subsequent increase in lactate concentration over time, since lipid levels may remain constant over time.
T1 maps were spatially homogeneous across the myocardium in all hearts (Figure 2A). Mean native T1 values in all hearts one hour after explantation was 727 ± 94 ms and it gradually increased over time to reach 765 ± 89 ms four hours after explantation. Such gradual increase may be explained by a progressive ischemic necrosis process (Figure 2B).
Conclusion:
This study proposes a protocol to assess myocardial viability in an ex vivo pig heart model using proton spectroscopy and T1 mapping. Preliminary results indicate a concomitant temporal increase in the lactate peak and myocardial T1 values over time, related to the severity of myocardial ischemia and necrosis process. We hypothesize that beyond a threshold to be determined, tissue viability may become compromised. Studies on a larger sample are needed before translating such viability evaluation into human grafts.
Example of voxel positioning at the interventricular septum (A) and the corresponding 1H spectrum (B). The fitting processing was designed to quantify lactate and myocardial lipid (1.3 ppm) and creatine (3 ppm).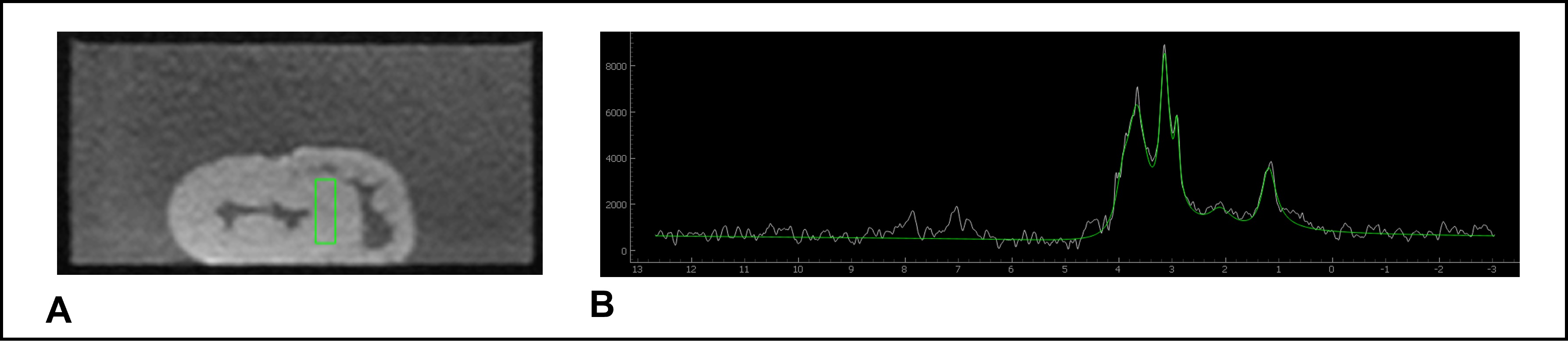
A representative T1 map of an explanted pig heart along with the myocardial contours (A), and T1 values evolution of the same heart over time (B).