Oral Case
CMR surveillance interval for individuals with pathogenic desmoplakin mutation - How soon is too soon?

Tania Ruiz M., MD
Assistant Professor of Medicine
Vanderbilt University Medical Center
Tania Ruiz M., MD
Assistant Professor of Medicine
Vanderbilt University Medical Center
Bruno B. Lima, MD, PhD
Assistant Professor of Medicine
Vanderbilt University Medical Center- JW
John A. Wells, IV, MD
Assistant Professor of Clinical Medicine
Vanderbilt University Medical Center 
Jeff Dendy, MD
Assistant Professor of Medicine
Vanderbilt University Medical Center
Vanderbilt- LL
Luke Laws, MD
Clinical Fellow
Vanderbilt University Medical Center - SM
Satish Mishra, MD
Clinical Fellow, Advanced Cardiac Imaging
Vanderbilt University Medical Center - SH
Sean G. Hughes, MD
Associate Professor of Clinical Medicine and Radiology Director, Cardiac MRI and Advanced Cardiac Imaging Fellowship
Vanderbilt University Medical Center
Presenting Author(s)
Primary Author(s)
Co-Author(s)
42 year old man with history of pathogenic desmoplakin (DSP) mutation and family history of sudden cardiac death developed arrhythmogenic cardiomyopathy diagnosed by Padua criteria three years after initial negative screening. Patient reported a single episode of unexplained syncope >20 years prior, but otherwise had remained asymptomatic with vigorous exercise. His father (at age 62) and paternal uncle (in his 40s) both had sudden cardiac death. On his initial screening, he had a normal MRI and signal-average ECG. Stress test showed frequent PVCs and a single 13-beat run of polymorphic VT. Electrophysiologic study did not elicit VT at that time. A follow-up CMR three years later demonstrated findings consistent with biventricular arrhythmogenic cardiomyopathy (ACM). A single-chamber ICD was implanted after these findings.
Diagnostic Techniques and Their Most Important Findings:
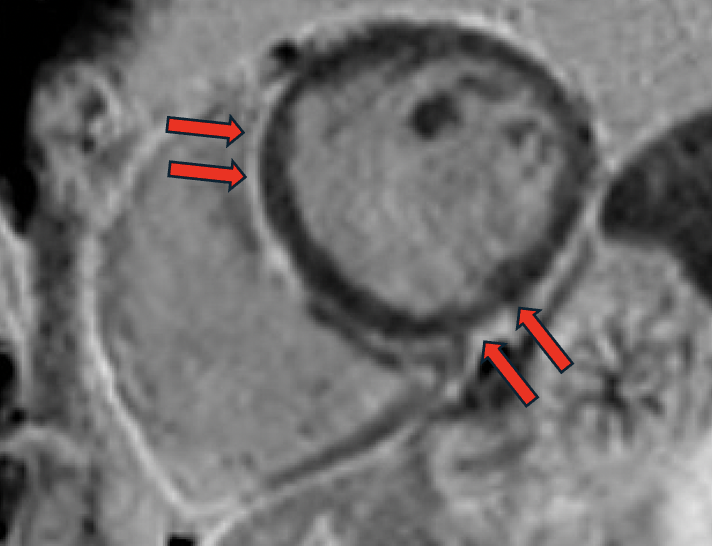
Both initial and follow-up CMRs were performed with the same comprehensive protocol, including cine and late gadolinium enhancement (LGE) in a 1.5T MRI scanner. Initial MRI showed mildly dilated LV (LVEDVi 108 mL/m2) with low-normal LV systolic function (LVEF 55%), top-normal RV size (RVEDVi 115 mL/m2) with normal RV systolic function (RVEF 53%), no myocardial LGE, and specifically no suggestion of ACM. At three-year follow-up, chamber sizes were relatively unchanged (LVEDVi 112 mL/m2 and RVEDVi 113 mL/m2), mild global reduction in LV systolic function (LVEF 51%), unchanged RV systolic function (RVEF 54%) but with regional RV dyskinesis in the basal free wall in a microaneurysm pattern (accordion sign) [Image 1] and focally in the RVOT and apex, focal LGE in the RV aspect of the interventricular septum [Image 2], subepicardial LGE in the basal anteroseptal and basal inferior LV walls in a stria pattern [Image 3], and normal T1/T2/ECV. On direct comparison to CMR three years prior, findings were now consistent with non-ischemic cardiomyopathy, favoring biventricular ACM.
Learning Points from this Case:
DSP is a desmosomal protein essential to cell-to-cell adhesion within cardiomyocytes. Several reports have linked the DSP gene to familial forms of arrhythmogenic cardiomyopathies (ACM). Three phenotypes have been recognized: right-dominant (ARVC), biventricular, and the most common left-dominant (ALVC). The main clinical presentations include extensive subepicardial LGE, disproportionate to the degree of LV dysfunction, in association with frequent ventricular ectopy and a propensity to life-threatening ventricular arrhythmia. The presence of any LV systolic dysfunction in DSP cardiomyopathy, particularly when associated with ventricular ectopy and LGE, indicates a substantial risk for severe ventricular arrhythmias. CMR follow-up at three years offered diagnostic and prognostic value when associated with pathogenic DSP mutation, but a shorter surveillance interval should be considered.
Four-chamber cine demonstrating extended focal bulging of the right ventricular wall (accordion sign).png)
Focal late gadolinium enhancement in the right ventricular aspect of the interventricular septum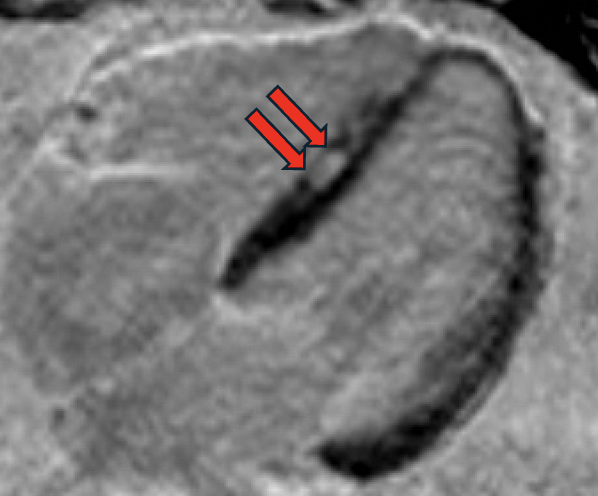
Subepicardial late gadolinium enhancement in the basal anteroseptal and basal inferior left ventricular walls in a stria pattern