Rapid Fire Abstracts
Arrhythmia Resolved 5D Flow Imaging Decodes the Effect of Arrhythmias on Flow (RF_TH_146)
- JB
Justin Baraboo, MSc
PhD candidate
Northwestern University - JB
Justin Baraboo, MSc
PhD candidate
Northwestern University - TN
Thara Nallamothu, BA
PhD Candidate
Northwestern University 
Elizabeth Weiss, PhD
MD/PhD student
Northwestern University- DD
David Dushfunian, MD
Clinical Research Associate
Northwestern University Feinberg School of Medicine - RP
Rod Passman, MD
Professor
Northwestern Memorial Hospital 
Dan Kim, PhD
Professor & Associate Vice-Chair for Research (Radiology)
Northwestern University
Northwesern
Daniel Lee, MD, MSc
Professor of Medicine and Radiology
Northwestern University Feinberg School of Medicine
Northwestern
Christopher W. Roy, PhD
Lecturer
Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Switzerland
Mathias Stuber, PhD
Full Professor
CIBM-CHUV-UNIL
Laussane University, Switzerland
Michael Markl, PhD
Professor
Northwestern University Feinberg School of Medicine
Presenting Author(s)
Primary Author(s)
Co-Author(s)
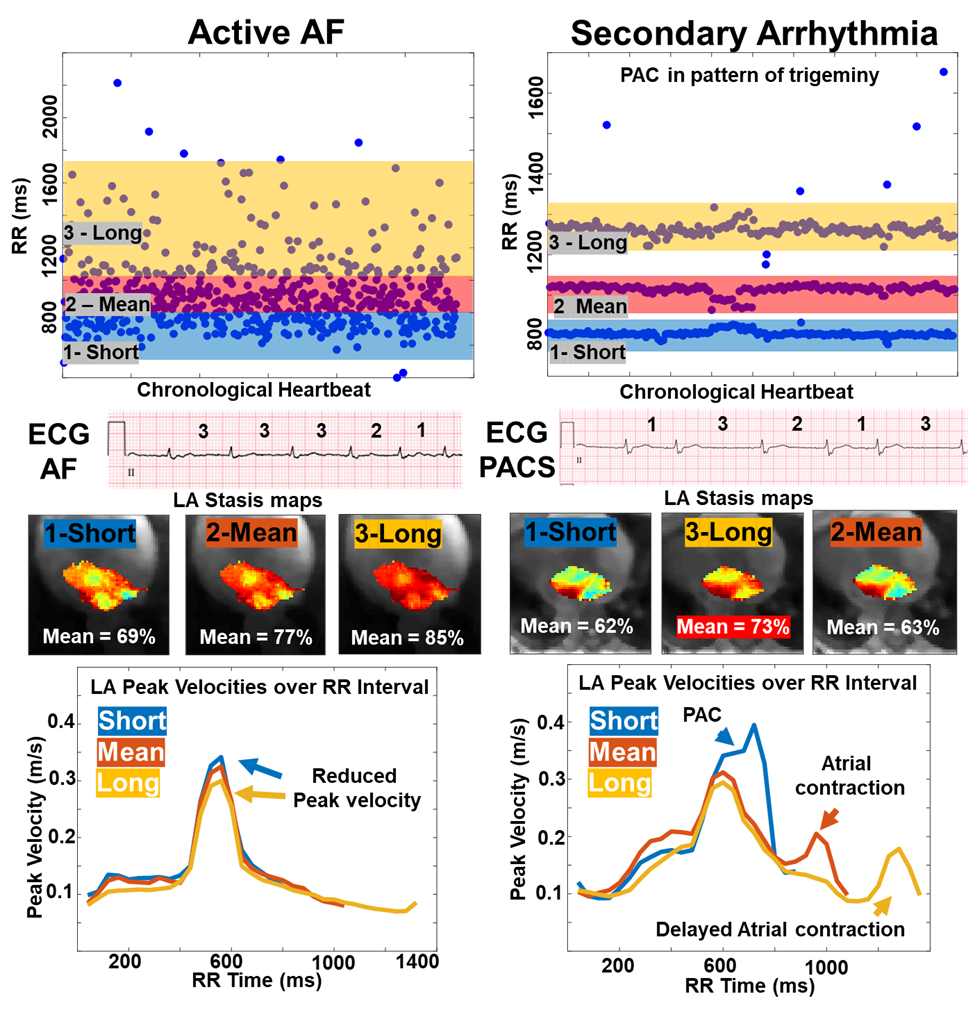
For patients in AF or a secondary arrhythmia, multiple time 3D time-resolved 4D flow data sets were reconstructed centered at a different heart rate. For atrial fibrillation, 3 non overlapping bins were reconstructed. For secondary arrhythmias, 2-3 image sets were reconstructed based on the discrete R-R intervals states, for example 3 in the PACs case shown. 5 of these patients had a clinical ECG within 6 months of the scan depicting the presence of secondary arrhythmia.
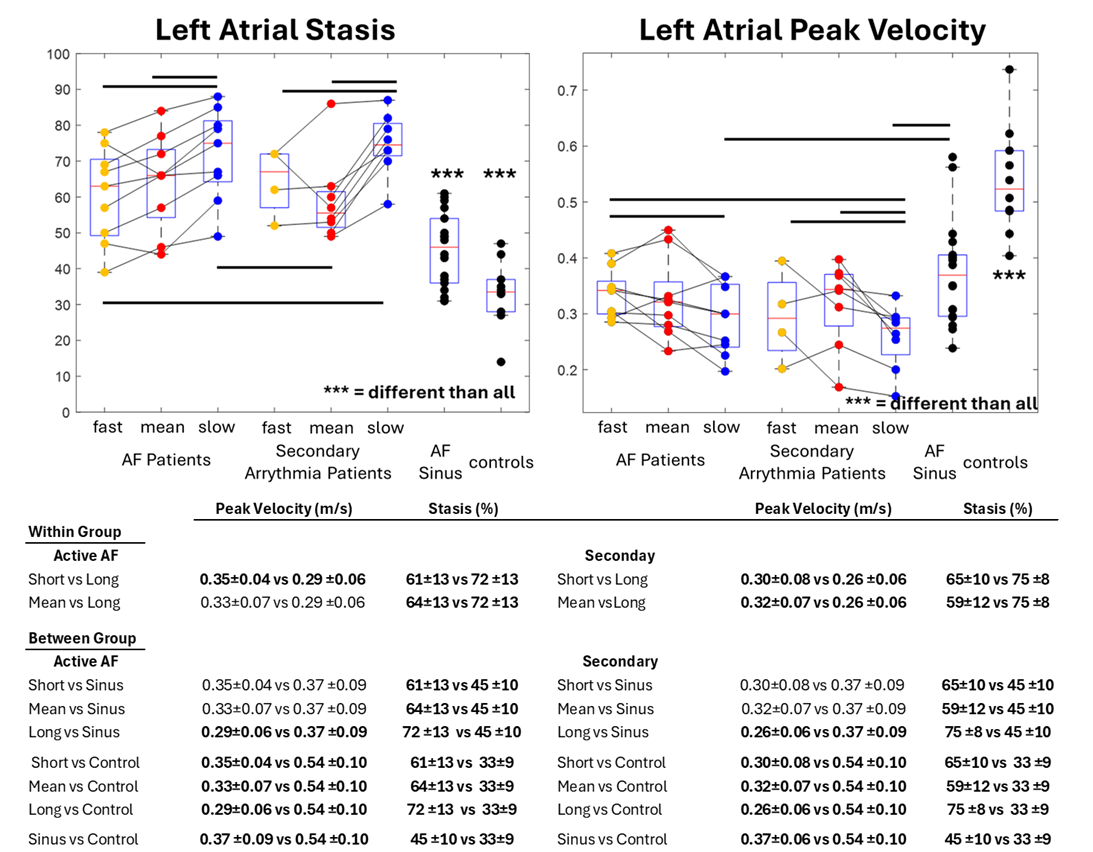
Pairwise within group and between group statistical testing. Atrial stasis was found to be increased for longer heartbeats within any arrhythmia with reduced peak velocity, displaying heart rate variable effects. Any arrhythmia also had lowered peak velocity and increased atrial stasis when compared to sinus (either for AF patients or controls). AF patients, at large, had lower peak velocities and higher stasis compared to controls.
Background:
Atrial fibrillation (AF) is associated with impaired left atrial (LA) hemodynamics, promoting thrombus formation and increased stroke risk. The main mechanisms causing these hemodynamic changes are thought to be driven by 1) AF induced stasis, and 2) long-term contributions of an underlying atrial myopathy (increased volume, fibrosis, etc.) [1]. Over 35% of AF patients also have non-AF, secondary arrhythmia (SA)[2]. The impact of SA on 3D LA blood flow dynamics is not well understood, especially in the setting of atrial myopathy. The studies purpose was to use a novel approach to imaging flow in patients with irregular heart rates to examine the impact of SA on LA hemodynamics in AF patients.
Methods:
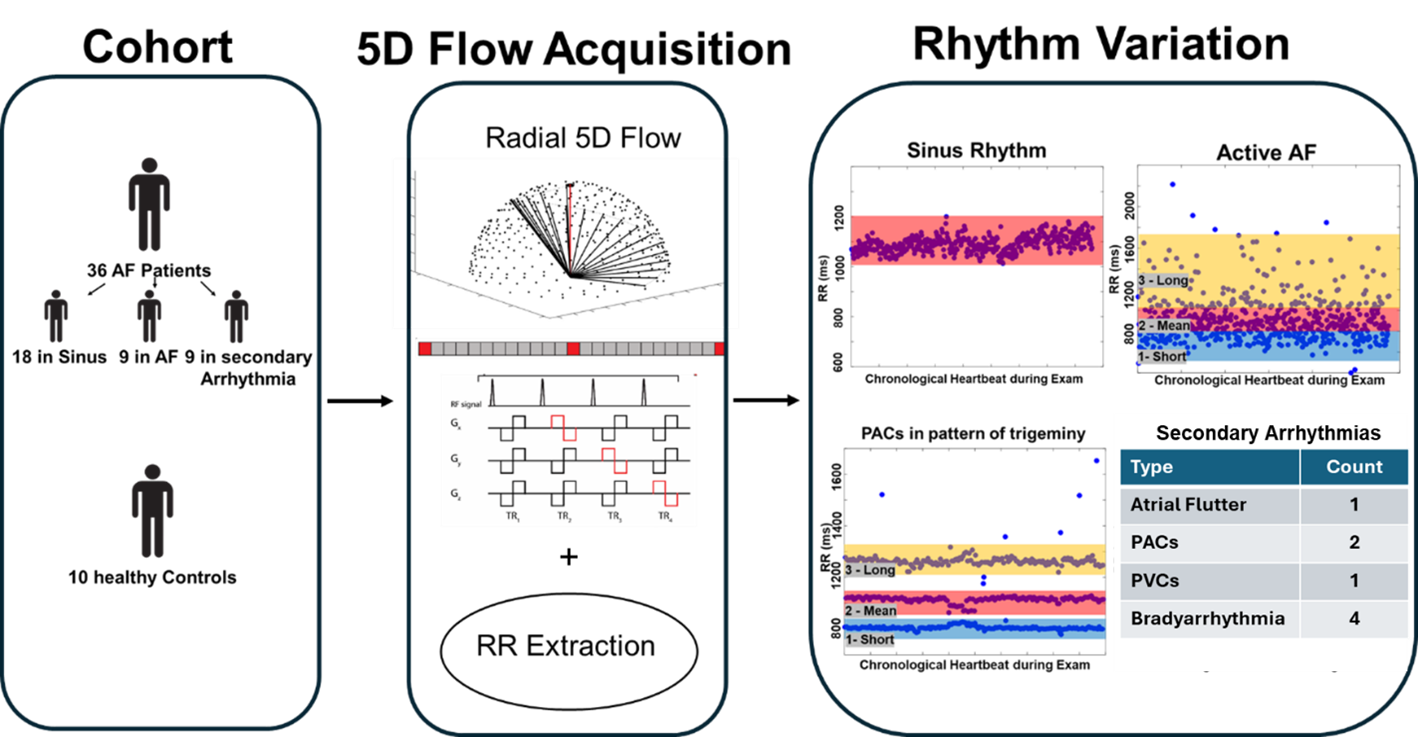
Arrhythmia resolved 5D flow MRI (5D=x,y,z,t,heart rate) [3] is a free running radial acquisition with 3-directional velocity encoding and inherent cardiac self-gating, utilizing compressed sensing for image reconstruction (fig 1). This framework allows for identification of individual heartbeat lengths (RRs) prior to reconstruction and the ability to dynamically segregate heartbeats into separate reconstructions.
36 AF patients (70±9 years, 9 female) and 10 healthy volunteers (40±22 years, 2 female) underwent MRI with arrhythmia resolved 5D Flow and were retrospectively enrolled.
Nine AF patients were in AF, having substantial RR variability and irregularity. Nine AF patients were classified with a non-AF SA, defined by 2-3 discrete heart rates (e.g. caused by premature atrial contractions (PACs) fig 1,2). 18 AF patients and the 10 healthy controls-maintained sinus rhythm.
For patients actively in AF, all k-space data for heart beats were sorted from shortest to longest RR-interval durations. Three non-overlapping RR-interval bins (short, mean, long RR duration) were reconstructed, resulting in 3 x 3D time-resolved 4D flow data sets. For AF patients with SA, 2- 3 data sets were reconstructed centered at each of the 2-3 discrete heart rates. Subjects in sinus rhythm had one data set reconstructed.
For each RR-resolved 4D flow data set, data analysis included eddy current correction, 3D LA segmentation, and calculation of LA blood stasis (% of cardiac time frames with LA velocities < 10 cm/s) and LA peak velocities per cardiac frame (fig2, stasis maps, LA peak curves). Mean LA stasis and overall LA peak velocities were quantified.
Results:
Detailed in fig 3, heart rate variable changes on LA flow were seen in any arrhythmia, demonstrating increased stasis (active AF: long RR 72 ± 13% vs short RR 61 ± 13% and mean RR 64 ± 13%, both p < 0.01; SA: long RR 75 ± 8% vs short RR 65 ± 10% and mean RR 59 ± 12%, both p < 0.01) and reduced peak velocities (active AF: long RR 0.29± 0.06 m/s vs short RR 0.35 ± 04 m/s, p = 0.009; SA: long RR 0.26 ± 0.06m/s vs short RR 0.30 ± 0.08 m/s and mean RR 0.32± 0.07 m/s, both p < 0.05).
AF patients in any arrhythmia exhibited higher LA stasis across all heartbeat durations compared to AF patients in sinus rhythm (45 ± 10%, all p < 0.01). All AF patients had increased stasis compared to healthy controls (33 ± 9%, all p < 0.006). Peak velocities during longer heartbeats in AF or a SA were reduced compared to AF patients in sinus (0.37± 0.09 m/s, both p < 0.02). All AF patients had lower peak velocities vs. controls (0.54± 0.1 m/s, all p < 0.001).
Conclusion: Our findings show that arrythmia resolved 5D flow MRI can successfully resolve LA blood flow changes across different heartbeats in AF patients with variable R-R intervals. Hemodynamic impairment) was similar for both arrhythmia groups and varied in intensity in different heart rates.
36 patients and 10 healthy controls underwent arrhythmia resolved 5D flow MRI (5D=x,y,z,t,heart rate), a free running radial acquisition with 3-directional velocity encoding and inherent cardiac self-gating. R-R intervals are extracted prior to image reconstruction. 18 AF patients and 10 controls exhibited sinus rhythm, 9 AF patients were actively in AF, and 9 were in a secondary arrhythmia. 
For patients in AF or a secondary arrhythmia, multiple time 3D time-resolved 4D flow data sets were reconstructed centered at a different heart rate. For atrial fibrillation, 3 non overlapping bins were reconstructed. For secondary arrhythmias, 2-3 image sets were reconstructed based on the discrete R-R intervals states, for example 3 in the PACs case shown. 5 of these patients had a clinical ECG within 6 months of the scan depicting the presence of secondary arrhythmia.
Pairwise within group and between group statistical testing. Atrial stasis was found to be increased for longer heartbeats within any arrhythmia with reduced peak velocity, displaying heart rate variable effects. Any arrhythmia also had lowered peak velocity and increased atrial stasis when compared to sinus (either for AF patients or controls). AF patients, at large, had lower peak velocities and higher stasis compared to controls.

