P/CHD
Pathway to Two Ventricles: Role of CMR in Determination of Operative Candidacy in a Patient with Atrioventricular Septal Defect and Right Ventricular Origin of the Aorta with Failing Single Ventricle Physiology
- SB
Snigdha Bhatia, MD
Fellow
Children's Healthcare of Atlanta - SB
Snigdha Bhatia, MD
Fellow
Children's Healthcare of Atlanta 
Sassan Hashemi, MD
Research Scientist
Children's Healthcare of Atlanta- RL
Ronald Allen Ligon, MD
Assistant Professor of Pediatrics
Emory University - WM
William A. McEachern, MD
Assistant Professor of Pediatrics
Emory University/Children's Healthcare of Atlanta 
Timothy Slesnick, MD
Pediatric Cardiologist
Children's Healthcare of Atlanta
Hunter C. Wilson, MD
Assistant Professor of Pediatrics
Emory University
Presenting Author(s)
Primary Author(s)
Co-Author(s)
A 6-year-old girl with Trisomy 21 and complete atrioventricular septal defect (AVSD) with right ventricular origin of the aorta and pulmonary atresia status post bilateral bidirectional Glenn (BDG) procedure presented for CMR. Prior to her presentation at our center, she had failed Fontan completion secondary to refractory chylous effusions and had undergone multiple lymphatic interventions. The latter included complete thoracic duct occlusion secondary to intractable chylous effusions despite selective channel embolization. Given the lymphatic dysfunction and her current physiology, CMR was requested to evaluate candidacy for a 1.5 ventricle or biventricular repair.
Diagnostic Techniques and Their Most Important Findings:
Echocardiogram showed large atrial and ventricular components of the AVSD. There was an adequate left mural leaflet and mild atrioventricular valve (AVV) regurgitation (Figure 1).
CMR demonstrated adequate left and right ventricular end-diastolic volumes (64 cc/m2 and 71 cc/m2, respectively) when creating a baffled pathway from the left ventricle to the aorta. There was no chordal straddle across the AVSD. BDG anastomoses were unobstructed, although flow in the left pulmonary artery (LPA) was entirely retrograde. There was flow from the left pulmonary veins into the left atrium, implying a source of pulmonary blood flow other than antegrade LPA flow. The patient underwent cardiac catheterization where contrast injection in the left superior vena cava showed no antegrade flow into the LPA. Selective angiography of the LPA showed significantly abnormal distal vasculature and arborization of the left-sided lung segments (Figure 2), presumably secondary to lymphatic engorgement.
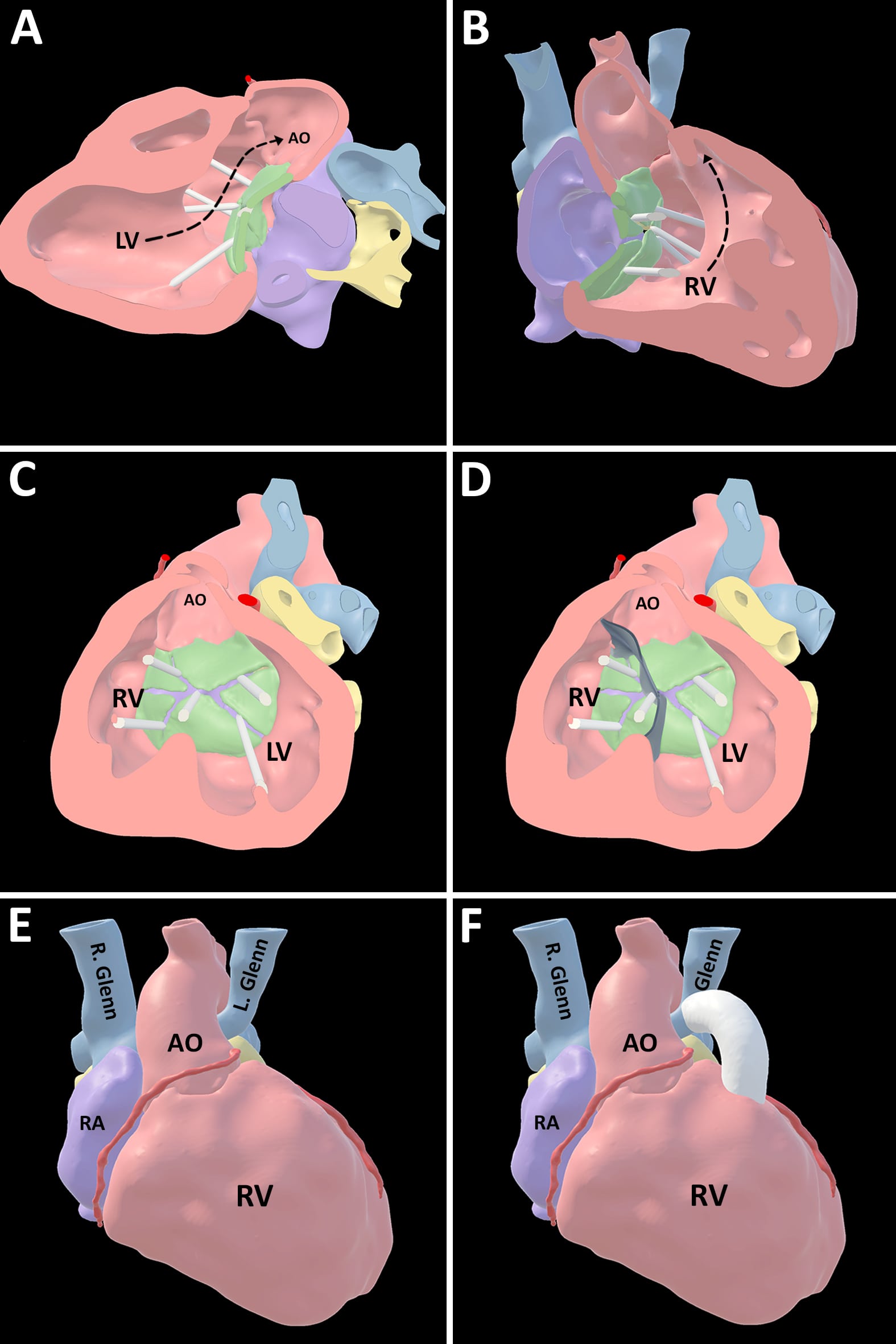
A 3-dimensional virtual model of the cardiac MRA dataset was created. The model was visualized in virtual reality where the pathway from the left ventricle to the aorta and a short infundibular segment with the potential to serve as the base for a right-ventricle-to-pulmonary-artery conduit were better conceptualized (Figure 3).
Learning Points from this Case:
Clinical and imaging data were reviewed in our institution’s patient management conference. Given significantly abnormal left lung vascular architecture and flow characteristics, both the current physiology and the potential for further single ventricular palliation were felt to be untenable. Therefore, in the setting of favorable characteristics on the 3D virtual model, the patient was deemed a candidate for biventricular repair. CMR supported surgical candidacy by 1) demonstrating adequate ventricular size accounting for a potential intracardiac baffle; 2) showing significantly abnormal flow characteristics in the LPA confirming poor candidacy for single ventricle palliation; and, 3) allowing construction of a virtual model that aided conceptualization of key anatomic considerations for a biventricular repair strategy.
Figure 1: Transthoracic echocardiogram demonstrated a Rastelli Type A AVSD with large primum ASD and inlet VSD components from an apical four-chamber view (Panel A, dotted line indicates plan of ASD and VSD) and typical atrioventricular valve morphology with an adequate left mural leaflet from subcostal sagittal view (Panel B, asterisks indicate superior bridging leaflet). RA: right atrium, LA: left atrium, RV: right ventricle, LV: left ventricle, AVSD: atrioventricular septal defect, VS: interventricular septum, LAVV: left atrioventricular valve, RAVV: right atrioventricular valve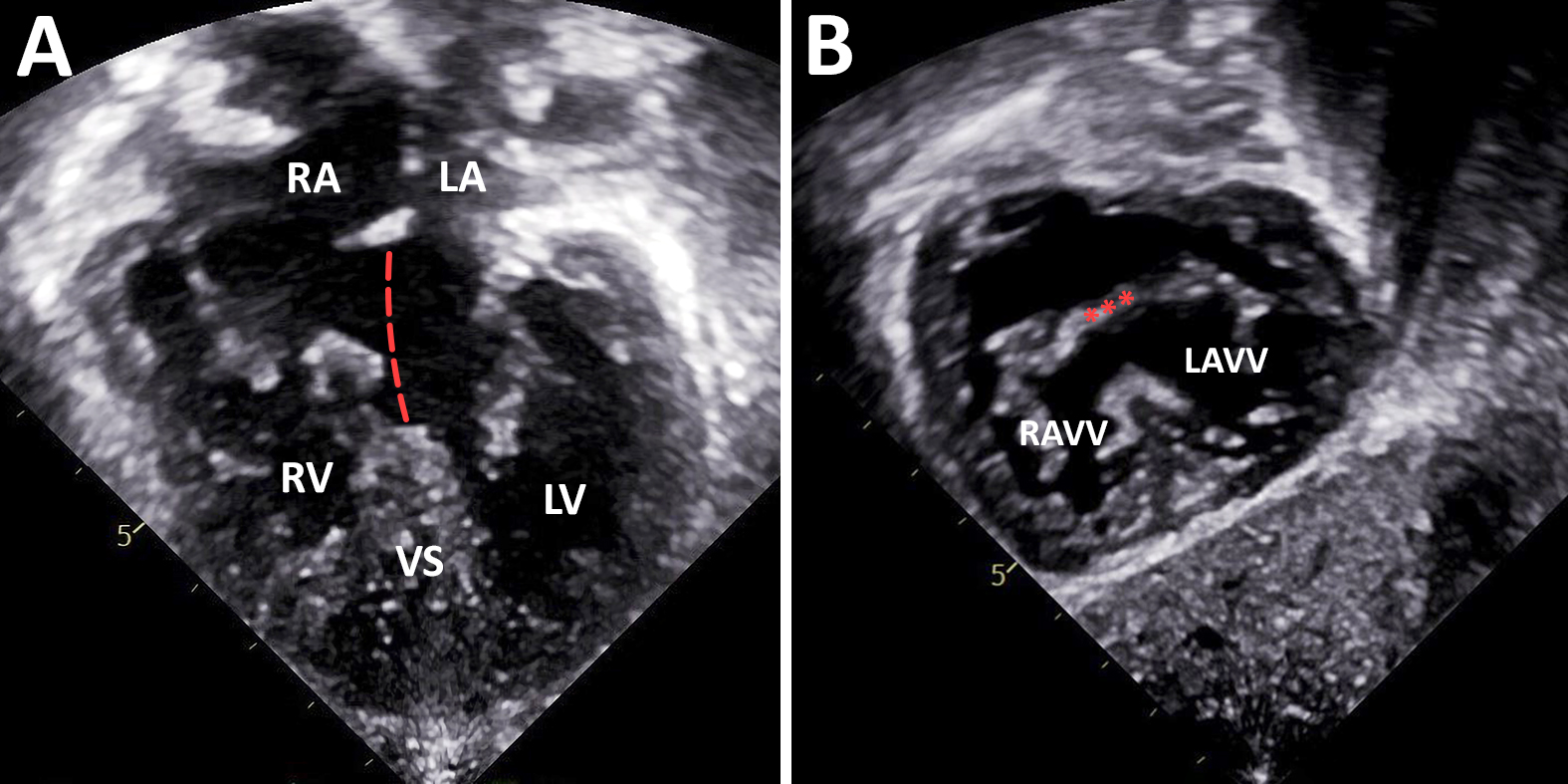
Figure 2: Cine CMR imaging demonstrated the potential pathway from the left ventricle to the aorta (Panel A). On coronal contrast-enhanced MRA imaging, bilateral bidirectional Glenns were patent although phase contrast imaging in the Glenn anastomoses and branch pulmonary artery segments demonstrated retrograde flow in the LPA (Panel B, arrows indicate direction of flow in each segment). Phase contrast imaging of the LPA showed rightward flow in both the LPA and left pulmonary veins (Panel C, dotted line indicates LPA, solid line indicates left pulmonary veins). A short infundibular segment was demonstrated left of the aorta, suggesting a potential base for a right-ventricle-to-pulmonary artery conduit (Panel D, dotted lines indicated potential conduit, asterisk indicates left coronary system). Angiograms demonstrated a decompressing vein but no flow into the LPA on injection in the superior vena cava (Panel E) and abnormal distal vasculature in the left lung on injection in the LPA suggestive of lymphatic abnormality (Panel F). LV: left ventricle, AO: aorta, RV: right ventricle, RPA: right pulmonary artery, LPA: left pulmonary artery, RVOT: right ventricular outflow tract (infundibular segment), LAA: left atrial appendage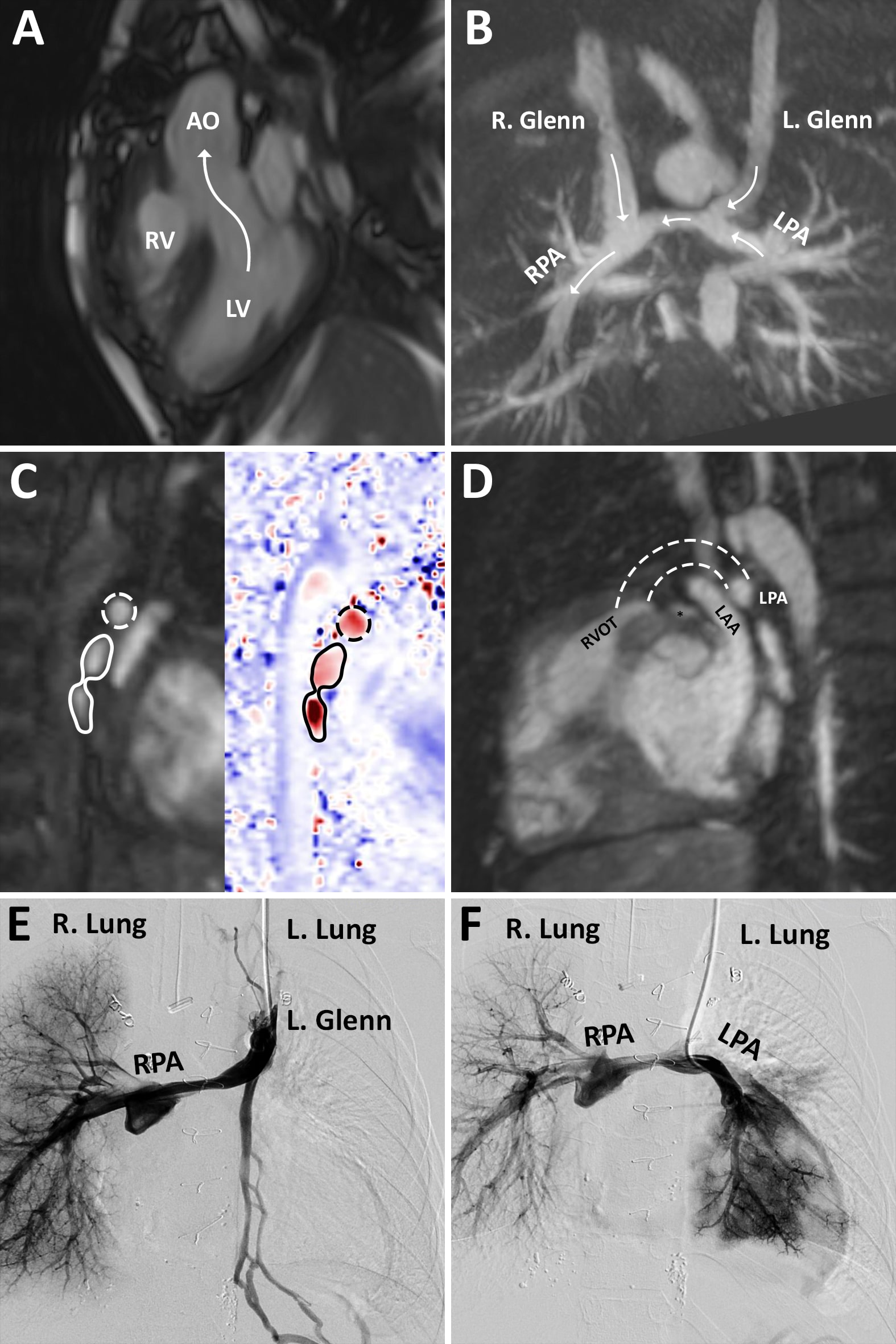
Figure 3: 3D virtual model showing: Potential pathway from LV to aorta (Panel A), potential pathway from RV to hypoplastic infundibular segment left of the aorta (Panel B), anatomy of atrioventricular septal defect (Panel C), model of an intracardiac baffle superimposed on the anatomy showing potential plane of ventricular septation (Panel D), position of aorta relative to bilateral bidirectional Glenn anastomoses (Panel E), and inclusion of a potential right-ventricle-to-pulmonary-artery conduit with base in the hypoplastic infundibular segment (Panel F). LV: left ventricle, AO: aorta, RV: right ventricle, LV: left ventricle, RA: right atrium