Clinical & Translation
Atorvastatin and left ventricular global circumferential strain during anthracycline-based chemotherapy
- VJ
Vencel Juhasz, MD
Research Fellow
Massachusetts General Hospital and Harvard Medical School - VJ
Vencel Juhasz, MD
Research Fellow
Massachusetts General Hospital and Harvard Medical School - TS
Thiago Quinaglia A. C Silva, MD, PhD
Assistant Cardiologist
State University of Campinas - UNICAMP, Brazil - ZD
Zsofia Drobni, MD, PhD
Cardiology fellow
Semmelweis University, Hungary - JH
Julius C. Heemelaar, MD
Research Fellow
Massachusetts General Hospital - DN
Donna S. Neuberg, PhD
Senior Lecturer
Dana-Farber Cancer Institute 
Yuchi Han, MD
Professor
The Ohio State University- BK
Bonnie Ky, MD
Professor
University of Pennsylvania 
Raymond Y. Kwong, MD
Professor of Medicine
Cardiovascular Division, Department of Medicine, Brigham and Women's Hospital, Boston, MA, USA- JJ
James Januzzi, MD
Chief Scientific Officer, Professor of Medicine
Massachusetts General Hospital, Baim Institute for Clinical Research - AA
Aarti Asnani, MD
Section Head
Beth Israel Deaconess Medical Center - RR
Robert A. Redd, MSc
Statistician
Dana-Farber Cancer Institute - NM
Negar Mousavi
McGill University, Canada
- MJ
Michael Jerosch-Herold, PhD
Associate Professor
Harvard Medical School - MS
Marielle Scherrer-Crosbie, MD, PhD
Professor
Hospital of the University of Pennsylvania 
Tomas G. Neilan, MD
Director, Cardio-Oncology Program
Massachusetts General Hospital
Presenting Author(s)
Primary Author(s)
Co-Author(s)
Anthracycline exposure may lead to a decline in the left ventricular ejection fraction (LVEF) and the development of heart failure. Left ventricular global circumferential strain (LV GCS) is an early marker of anthracycline-associated cardiotoxicity and carries prognostic value for asymptomatic individuals. Additionally, GCS is less influenced by loading conditions than LVEF. In the STOP-CA clinical trial, atorvastatin reduced the risk of a clinically significant decline in LVEF during anthracycline-based chemotherapy. However, whether atorvastatin can attenuate the decline in GCS from treatment with anthracyclines is not known.
Methods:
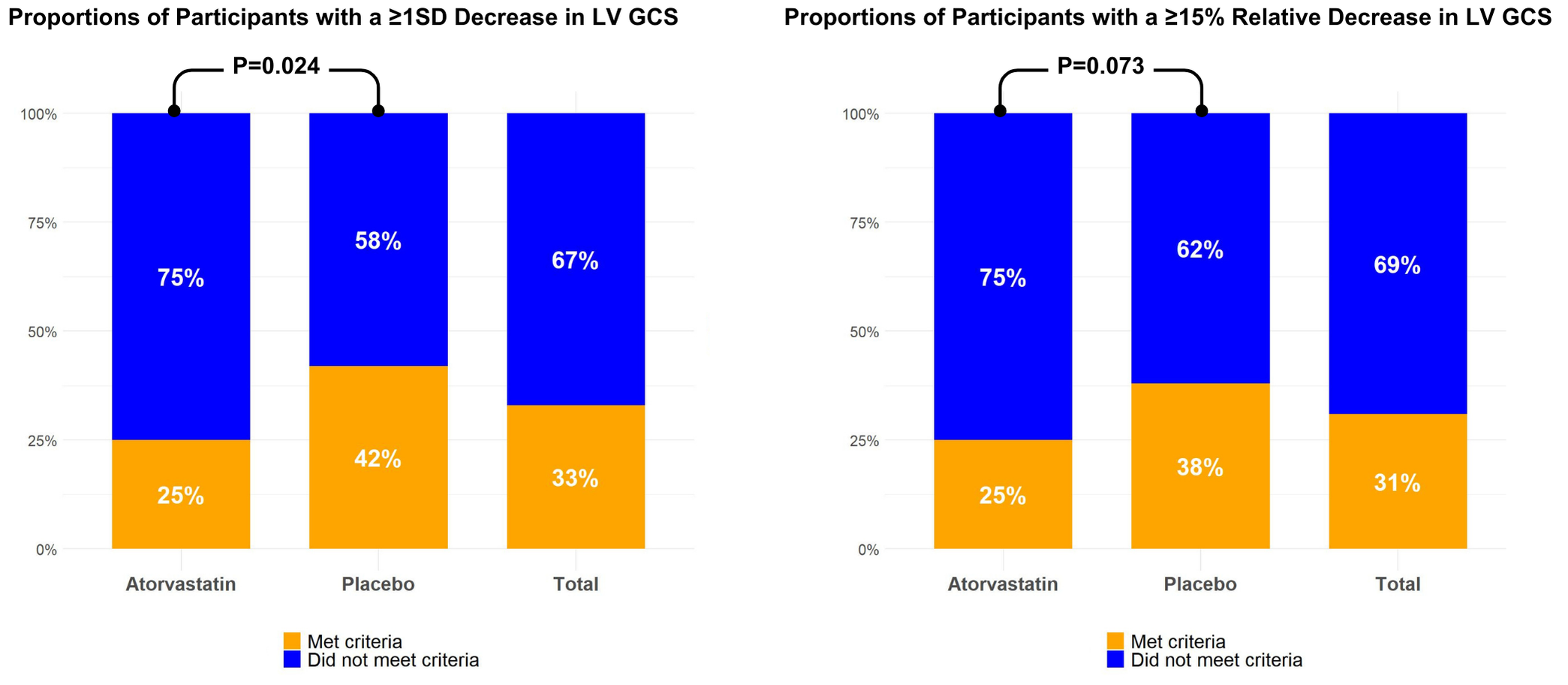
STOP-CA recruited 300 newly diagnosed lymphoma patients in nine centers across the US and Canada. Participants were randomized to atorvastatin (n=150) or placebo (n=150) for 12 months. Cardiac MRI was performed at baseline and 12 months. Short-axis cine sequences were contoured endo and epicardially in a basal, a mid-ventricular, and an apical slice to calculate LV GCS (Medis Suite v3.2/QStrain 2.0, Leiden, The Netherlands) with feature-tracking (FT). Due to the lack of established cut-offs, the primary outcome of interest was the proportion of participants in each group with a ≥1 standard deviation (SD) decrease in GCS. The secondary outcome of interest was the proportion of participants in each group with a ≥15% relative decrease in GCS, previously associated with subsequent cardiotoxicity.
Results:
Of the 300 participants, 177 (93 in the atorvastatin group, median age 52 years, 48% female) had paired data of LV GCS. The baseline LV GCS was similar between groups (21.9±3.1% for atorvastatin vs. 21.8±3.1% for placebo, P=0.73), while follow-up values were lower with placebo (20.2±3.3% vs. 19.3±2.7%, P=0.012). The proportion of participants with a ≥1SD (3.2% unit) decrease in the absolute LV GCS values was significantly lower in the atorvastatin group (25% vs. 42%, OR 0.46, CI 0.24-0.87, P=0.024). There was a numerically but not statistically significant lower proportion of participants with a ≥15% relative decrease in GCS (25% vs. 38%, P=0.073) and a smaller absolute GCS decline in the atorvastatin group (-1.7±3 vs. -2.5±3.4%, P=0.085). Moreover, there were associations between the decrease in LVEF and LV GCS, where a ≥10% LVEF decrease at follow-up was associated with a 2.6% decrease in GCS (CI -3.67, -1.52, P< 0.001). Similarly, participants with a ≥10% decrease in LVEF had a 4.94 higher odds of meeting the primary GCS endpoint (CI 2.33, 10.77, P< 0.001).
Conclusion:
Compared to placebo, atorvastatin reduced the risk for a significant decline in cardiac MRI FT-derived LV GCS in lymphoma patients undergoing anthracycline-based chemotherapy. The results support the cardioprotective effects of atorvastatin during anthracycline therapy and provide mechanistic insight into its mechanisms. Further investigation is warranted regarding the clinical utility of tracking changes in LV GCS during anthracycline therapy.
Study flowchart.png)
Barcharts representing proportions of participants meeting the primary and secondary LV GCS endpoints