Early Career
3D Quantification of Maximal Left Ventricular Wall Thickness: Associations with Global Fibrosis Burden and Future Adverse Outcomes in Hypertrophic Cardiomyopathy
- RM
Rylan Marianchuk, BSc
Masters Student
University of Calgary, Canada - RM
Rylan Marianchuk, BSc
Masters Student
University of Calgary, Canada - FH
Fereshteh Hasanzadeh, MSc
Masters Student
University of Calgary, Canada - TB
Talia A. Beckie, BEng
Cardiac Imaging Systems Engineer
Libin cardiovascular Institute, University of Calgary, Canada - CW
Connor J. White, N/A
High School Student
Libin Cardiovascular Institute, University of Calgary, Canada - KA
Kate Aspinall
Undergraduate student, Queens University
Libin Cardiovascular Institute, University of Calgary, Canada - JF
Jacqueline Flewitt, MSc
Manager of Strategic Partnerships
Libin Cardiovascular Institute; University of Calgary, Canada - SR
Sandra Rivest, RN
Research Coordinator
Libin Cardiovascular Institute; University of Calgary, Canada - GP
Grant Peters, MD
Clinical Assistant Professor
Libin Cardiovascular Institute, University of Calgary, Canada - AH
Andrew G. Howarth, MD, PhD
Associate Professor
Libin Cardiovascular Institute; University of Calgary, Canada - CL
Carmen P. Lydell, MD
Clinical Associate Professor
Libin Cardiovascular Institute; University of Calgary, Canada - RM
Robert J.H. Miller, MD
Clinical Assistant Professor
Libin Cardiovascular Institute; University of Calgary, Canada - LK
Louis Kolman, MD
Clinical Assistant Professor
Libin Cardiovascular Institute; University of Calgary, Canada 
Dina Labib, MD, PhD, FSCMR
Associate Scientific Director, Personalized Diagnostics Program; Adjunct Assistant Professor
University of Calgary, Canada
James A. White, MD
Professor
Libin Cardiovascular Institute; University of Calgary, Canada
Presenting Author(s)
Primary Author(s)
Co-Author(s)
Maximal left ventricular (LV) wall thickness (MWT) is a well recognized and ubiquitously sought marker of phenotypic severity in hypertrophic cardiomyopathy (HCM). This marker has and continues to be incorporated into contemporary risk prediction models in this population. However, this marker suffers from poor intra- and inter-observer variability related to its subjective visual localization and manual quantification. Additionally, the relationship between MWT and other high-risk phenotypic markers, such as replacement fibrosis, have been poorly studied. In this study, we explore the value of automated, deep learning-based 3D-shape phenomics to regionally identify and quantify MWT without need for human interaction. The association of this marker, described at both a global and segmental level, with global fibrosis burden was then described. Finally, independent associations of 3D MWT with future clinical outcomes was determined.
Methods: A random sub-group of 359 patients with HCM who underwent CMR were selected from the Cardiovascular Imaging Registry of Calgary (CIROC). 3D shape phenomics was executed using locally developed software, performing segmentation of long and short-axis cines followed by image alignment and bi-layer LV chamber meshing. The latter generates a standardized 3D shape model of endocardial and epicardial surface nodes structured as a finite element model of 456 hexahedral elements; subsequently resolved to 16 AHA segments (Figure 1). LV 3DMWT was calculated segmentally as a maximum of each segment’s constituent elements with this highest value chosen as global 3DMWT. Signal threshold-based analysis of LGE images (≥ 5 SD reference myocardium) was performed using cvi42 to calculate segmental and global fibrosis burden. Patients were followed for the composite outcome of all-cause mortality, heart failure hospitalization, ventricular arrhythmia, new-onset atrial fibrillation, and survival of sudden cardiac arrest. A multivariable Cox model was constructed to test the association between global 3DMWT and the primary outcome, adjusting for age, sex, HCM morphology, and global LGE fibrosis burden.
Results:
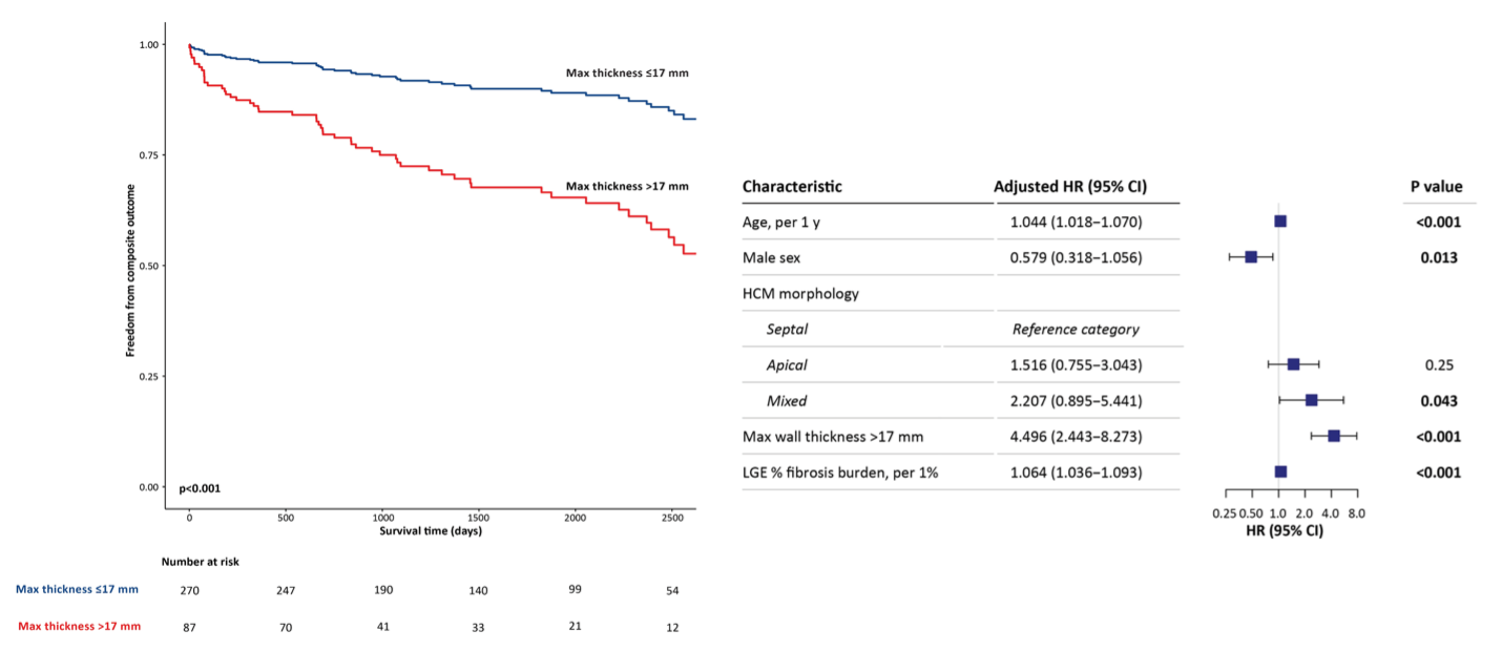
A total of 5,744 myocardial segments were analyzed. LV 3DMWT showed weak associations with LGE fibrosis burden at both a global (r=0.18, p=0.035) and segmental (r=0.17, p=0.07) level, as shown in Figure 2. In a multivariable Cox model, global 3DMWT was independently associated with the composite outcome with an adjusted HR of 1.10 per 1mm (95% CI: 1.04-1.17; p=0.001). Using a survival-based optimal cut-point of 17 mm for 3DMWT, patients above this threshold experienced a 4.5 fold increased risk of the composite outcome versus those below; adjusting for age, sex, global LGE fibrosis burden, and HCM morphology sub-type (HR 4.50 [2.44-8.27] p< 0.001; Figure 3, right panel). Adjusted Kaplan-Meier curves are shown in Figure 3, left panel).
Conclusion:
This is the first study exploring associations of LV 3DMWT with fibrosis burden and future clinical outcomes in patients with HCM. We identified that MWT is only weakly associated with replacement fibrosis burden, however is strongly and independently associated with future clinically relevant outcomes. Accordingly, these markers should be considered of independent value for risk modelling. Expanded study in a larger cohort is planned to elucidate independent value for arrhythmic versus non-arrhythmic outcomes.
Derivation of 3D cardiac shape models. Left: Automated multi-axial segmentation of left ventricular endocardial and epicardial boundaries followed by automated correction for breath-hold malalignment. Middle: 3D finite element mesh model generation with 456 hexahedral elements. Right: Calculation of element level wall thickness and volumes used for estimation of both segmental and global maximal values.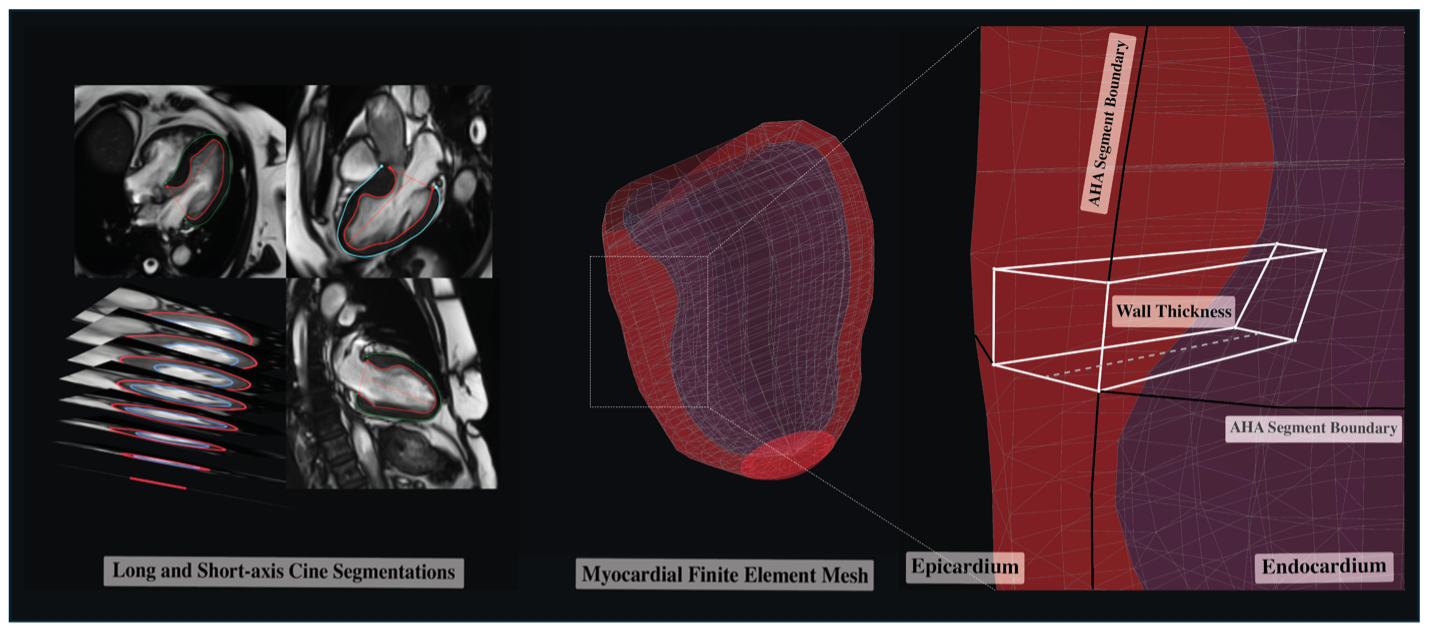
Scatter plots demonstrating weak associations between 3DMWT and volume and LGE fibrosis burden (%) at both a global (left) and segmental (right) level. 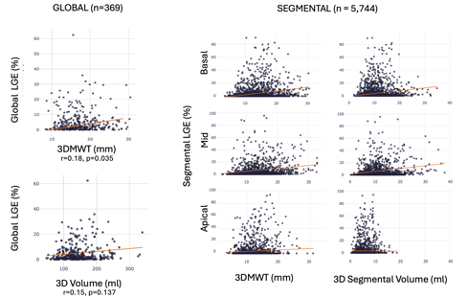
Adjusted Kaplan-Meier survival curve for freedom from the composite outcome for LV 3DMWT dichotomized at the optimal cutpoint of 17 mm, adjusted for age, sex, HCM sub-type and global LGE fibrosis burden (left). Forest plot (right) show results of multivariable Cox model.