Science Sessions
Dynamic myocardial T2* relaxometry in a mouse model of HCM at 9.4T
- SS
Shahriar Shalikar, MSc
PhD Student
Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany., Germany - SS
Shahriar Shalikar, MSc
PhD Student
Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany., Germany - OL
Oumaima Laghzali, MSc
PhD Student
Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany. Charité—Universitätsmedizin Berlin, Germany. DZHK (German Centre for Cardiovascular Research), partner site Berlin, Berlin, Germany, Germany - SL
Siqin Liu, MD
Dr.med student
Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany; DZHK (German Centre for Cardiovascular Research), partner site Berlin, Germany; Charité-Universitätsmedizin Berlin, Berlin, Germany;, Germany - SL
Sandra Lehmann, MSc
Researcher
abc, Germany - SW
Sonia Waiczies, PhD
Senior researcher
Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany., Germany - LC
Lucie Carrier, PhD
Prof.Dr.
Department of Experimental Pharmacology and Toxicology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany. DZHK (German Centre for Cardiovascular Research), partner site Hamburg/Kiel/Lübeck, Hamburg, Germany., Germany .jpeg)
Hsin-Jung Randy Yang, PhD
Assistant Professor
Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Thoralf Niendorf, PhD
Prof.Dr.
Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany. Experimental and Clinical Research Center, Charite Medical Faculty and the Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany. DZHK (German Centre for Cardiovascular Research), partner site Berlin, Berlin, Germany., Germany- MK
Min-Chi Ku, PhD
Senior researcher
Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany. DZHK (German Centre for Cardiovascular Research), partner site Berlin, Berlin, Germany, Germany
Presenting Author(s)
Primary Author(s)
Co-Author(s)
The Inversion Recovery Late Gadolinium Enhancement (IR-LGE) imaging method provides high contrast for depicting myocardial lesions. Still, inappropriate inversion time (TI) settings can lead to incomplete examinations, burdening the examiner. Although it has been reported that synthetic LGE can be created from T1 mapping, it is difficult to image simultaneously and compare images. Using dynamic T1 mapping for pharmacokinetic analysis makes it possible to create T1 maps from concentrations at any given time, allowing image evaluation with precisely matched imaging times. This study aims to investigate whether synthetic IR-LGE created from T1 maps adjusted to the post-imaging time of actual IR-LGE scan using pharmacokinetic analysis can achieve image quality, contrast, and lesion detection equivalent to actual IR-LGE.
Methods:
50 participants who had planned CMR for myocardial evaluation (61 years, IQR 48,73) were included in this study. Actual LGE imaging (aLGE) was performed 10 minutes after contrast medium injection following TI-scout image acquisition for all participants using the inversion recovery method. Additionally, contrast agent concentrations at each time point were calculated from sequentially acquired T1 maps, and the T1 map 10 minutes after contrast administration was computed using a myocardial contrast agent concentration pharmacokinetic analysis model. Synthetic inversion recovery LGE images (sLGE) were created with TI ranging from 220 ms to 400 ms based on pharmacokinetics-derived T1 maps. Heart rate during scan was also reflected in the sLGE signal values. The null point TI of the myocardium was recorded in the Look-Locker TI-scout image and sLGE. Using sLGE images with the same TI as for aLGE imaging, sLGE and aLGE images were evaluated for image quality (5 points: 1; non-diagnostic, 5; excellent), pattern (endocardial, epicardial, mesial, ischaemic, transmural, none) and contrast ratio between LGE lesion and remote myocardium.
Results: The TIs of the null myocardium point were 320±25msec with TI-scout and 316±22msec with sLGE (p=0.01). The image quality score was higher for sLGE compared to aLGE (4.7 vs. 4.3, p=0.03). The enhancement patterns were classified for aLGE and sLGE as follows: endocardium 3 vs. 3, epicardium 9 vs. 11, mid 22, ischemic 1 vs. 1, transmural 2 vs. 2, none 13 vs. 11, respectively. One non-diagnostic case was identified in aLGE. There was one case each where epi and mid were determined in sLGE but classified as none in aLGE. The contrast ratio of LGE was higher for sLGE compared to aLGE (0.70 vs. 0.58, p< 0.01).
Conclusion: Synthetic LGE created from T1 maps aligned with LGE imaging time using pharmacokinetic analysis can be used for myocardial lesion evaluation equivalent to IR-LGE.
46-years-old man, with dilated cardiomyopathy The left panel shows conventional inversion-recovery LGE (TI=310 msec), the right panel shows synthetic LGE from a pharmacokinetics-derived T1 map (TI=310 msec) A mid-wall linear high-intensity lesion is shown in both images.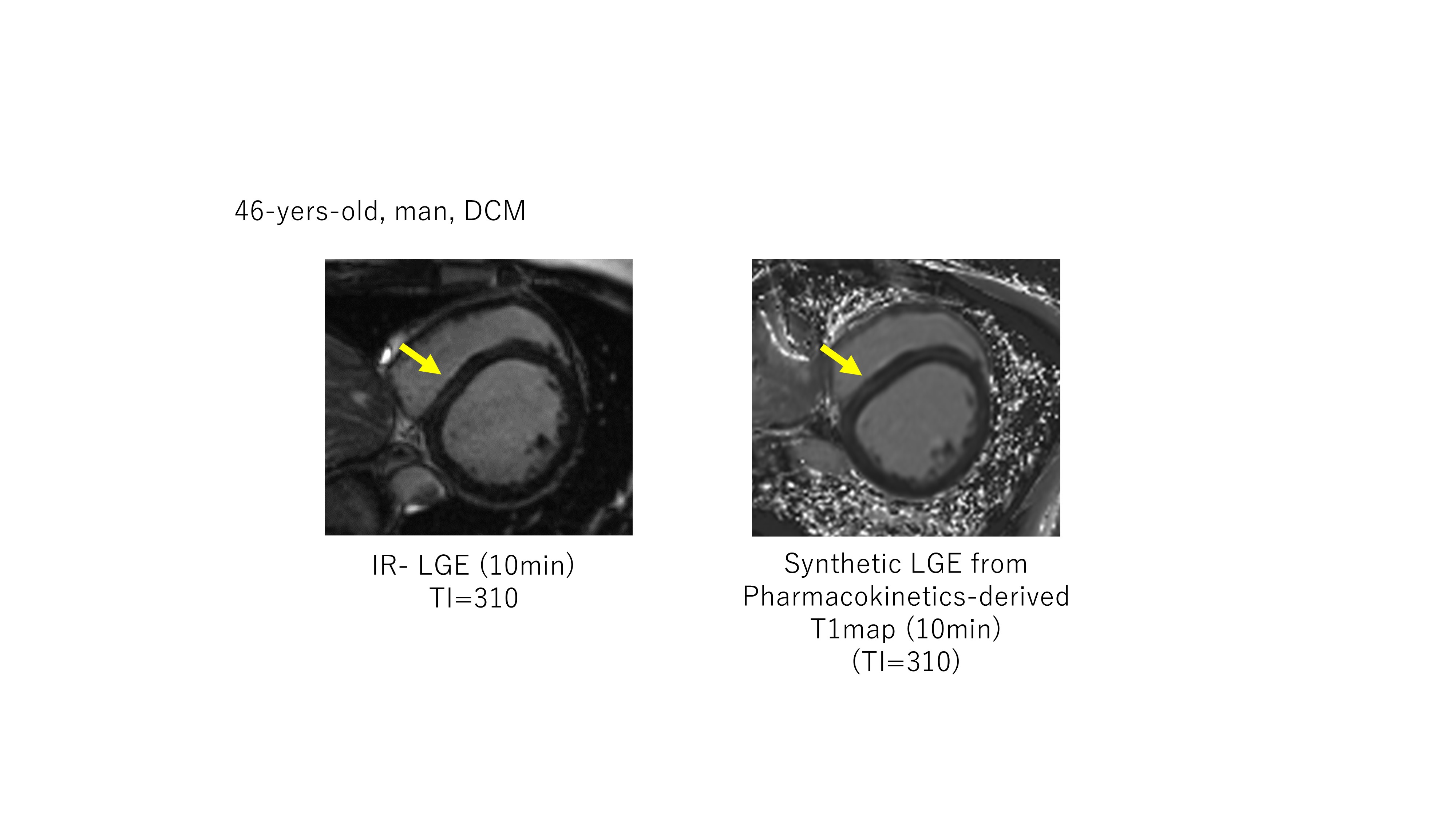
LGE pattern difference between IR-LGE and synthetic LGE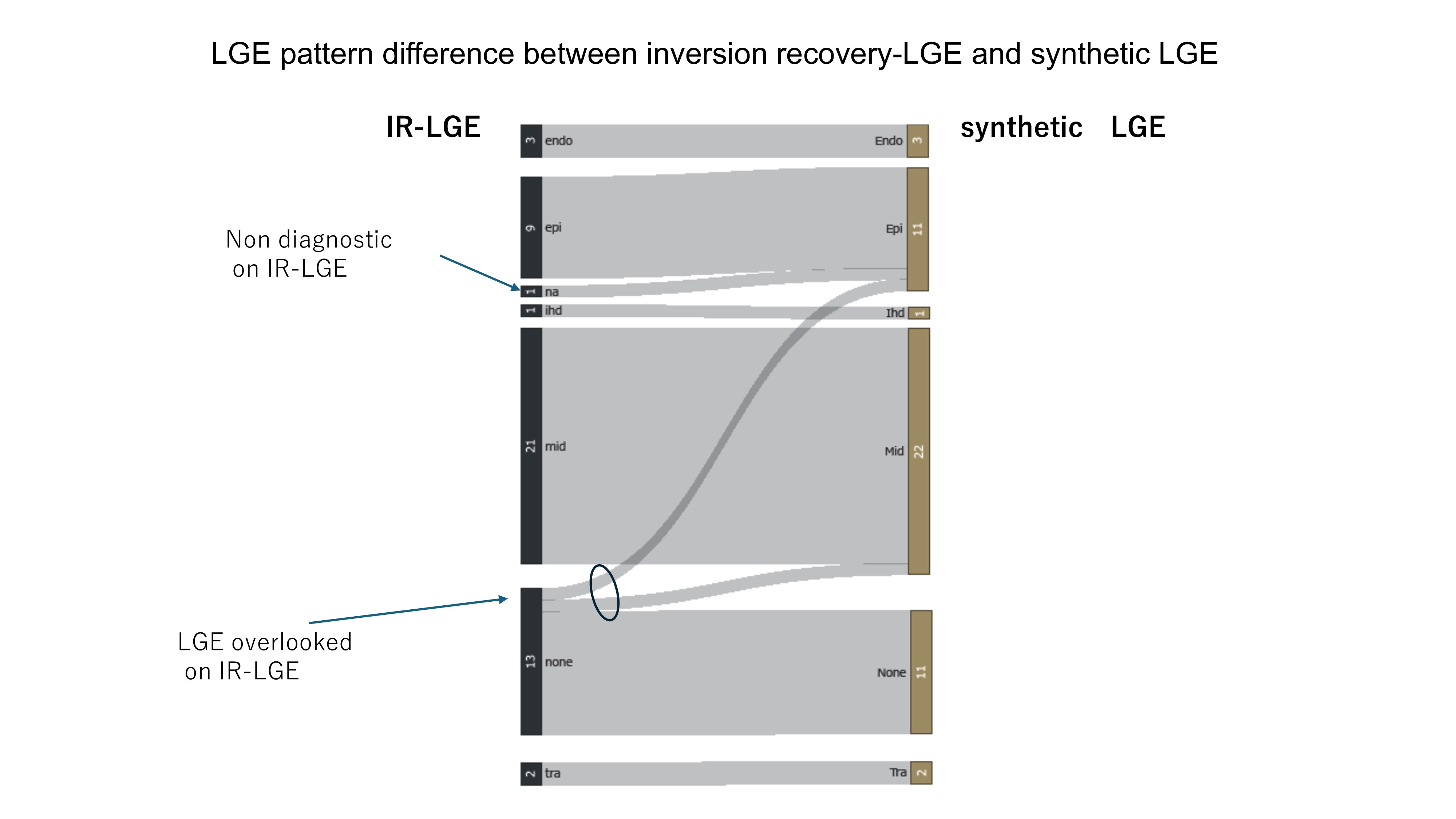
Figure 1. Data Acquisition and Reconstruction scheme used for whole cardiac cycle T2*-mapping of the mouse heart. MRI data acquisition is performed continuously in synchronization with the pulse-oximetry and with the scanner's TTL trigger signal. Following data collection, retrospective sorting based on recorded signals is performed. Each k-space line is assigned to the corresponding cardiac phase and then averaged to yield 10 cardiac phases, each with 7 echoes. T2* maps are generated via pixel-wise mono-exponential fitting of the T2* signal decay.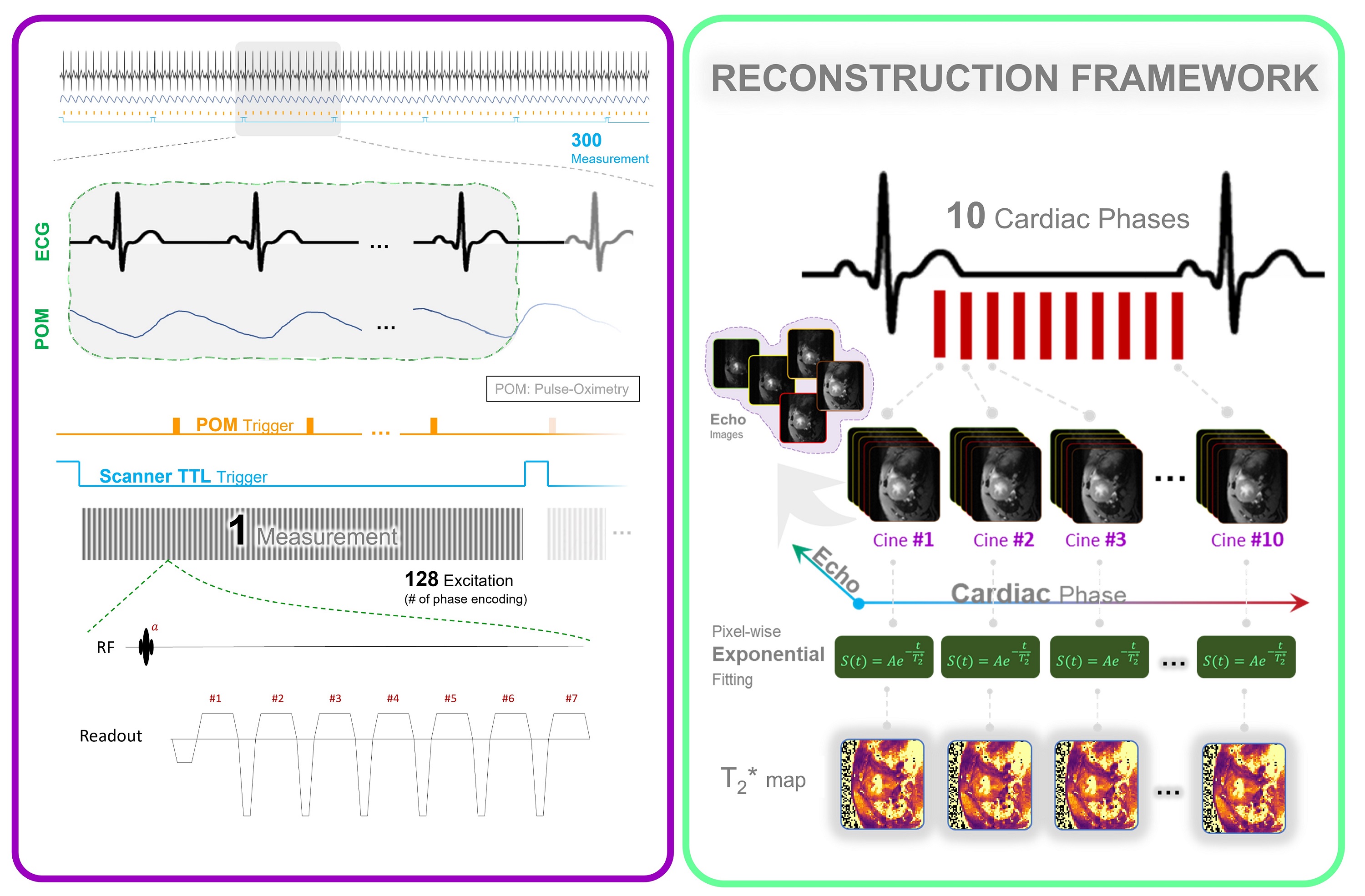
Figure 2. T2* phantom study and qualitative comparison and quantitative analysis with reference value. A) Qualitative comparison of T2* map. An exemplary first echo image (TE= 1.5) of six iron concentrations reconstructed by the retrospective binning approach together with T2* maps of the phantom obtained for conventional MGE and retrospective binning MGE. B) Regression and Bland-Altman analysis. The retrospective binning approach shows strong correlation with the reference method, as indicated by R2. The dotted line shows identity line, while the solid line illustrates the linear regression fitting. Bland-Altman plot also depict the comparison of the reference method with the retrospective binning approach. The dashed lines indicate a confidence level of 95%, while the continuous lines depict the average percentage variances. .jpg)
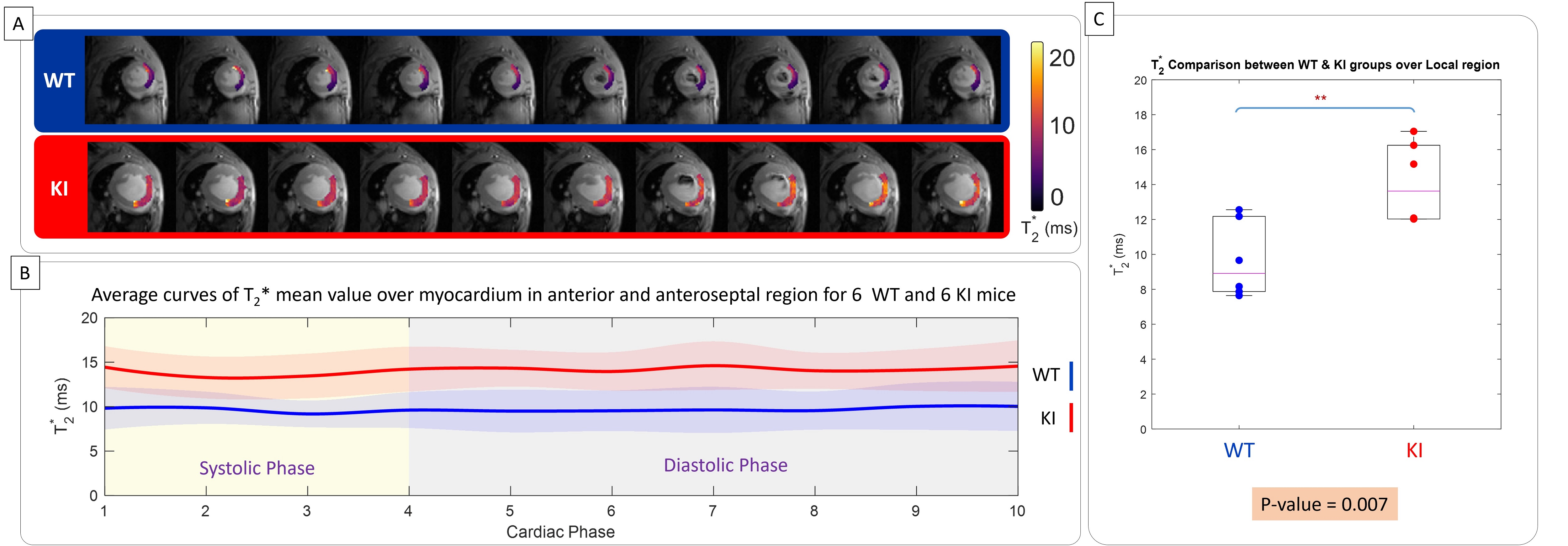
Figure 3. In vivo myocardium T2* measurement covering the whole cardiac cycle in WT and in HCM mice in the region of no off-resonance artifact. A) Representative regional T2* maps superimposed to anatomic images obtained for 10 cardiac phases for a male WT control mice (top) and for a female mybpc3-ki mice (bottom). B) Comparison of T2* between WT controls and mybpc3-ki HCM mice reveals elevated T2* values in the HCM mice across all cardiac phases (n = 12 mice). There is a T2* variation across the cardiac cycle. C) The box-plot of mean myocardial T2*, averaged across the cardiac cycle for each mouse, reveals significantly higher values in the specified region of HCM mice compared to controls (n=6, 3 females). Statistical significance was assessed using unpaired Student's t-tests, with p-values provided numerically.