Rapid Fire Abstracts
Free-breathing Single-Shot Late Gadolinium Enhancement Imaging with Variable Inversion Time (vTI LGE) for Imaging of Scar and Lipomatous Metaplasia (RF_TH_159)
- OD
Omer Burak Demirel, PhD
MRI Clinical Scientist
Philips - OD
Omer Burak Demirel, PhD
MRI Clinical Scientist
Philips 
Alexander Schulz, MD
Dr.
Harvard Medical School / BIDMC, Germany
Fahime Ghanbari, MD
Fellow
Harvard medical school, Beth Israel Deaconess Medical Center- PP
Patrick Pierce, BSc
MRI Technologist
Beth Israel Deaconess Medical Center - SJ
Scott Johnson
MRI Tech
Beth Israel Deaconess Medical Center - JR
Jennifer Rodriguez
Clinical Trials Specialist
Beth Israel Deaconess Medical Center - KA
Kathryn Arcand, RN
Nurse
Beth Israel Deaconess Medical Center - WM
Warren J. Manning, MD
Section Chief, Non-invasive Cardiac Imaging
Beth Israel Deaconess Medical Center/Harvard Medical School
Beth Israel Deaconess - RN
Reza Nezafat, PhD
Professor
Harvard Medical School
Presenting Author(s)
Primary Author(s)
Co-Author(s)
.png)
Figure 2: A) Initial GRAPPA reconstruction is followed by zero-padding and resolution enhancement using the generative adversarial inline neural network (REGAIN), which reconstructs images to a spatial resolution of 1.9 × 1.9 mm2 from 1.9 × 4.9 mm2. B) Imaging parameters of PSIR and REGAIN acquisitions are given in the table. 3.7-fold accelerated LGE images were acquired with 2-fold GRAPPA at 40% phase resolution, corresponding to a 210 × 68 matrix size, which was then zero-padded to 210 × 168. C) Representative free-breathing LGE images before and after image enhancement for three subjects with no LGE across six consecutive TIs. REGAIN reconstruction visibly improved image quality. The three patients achieved ideal nulling at 360, 400, and 340 ms, respectively. The third patient has subendocardial scars. D) Quantitative analysis of image sharpness using a reference-free blur metric (0: sharpest image quality, 1: blurriest image quality) showed that REGAIN-processed LGE images had significantly higher sharpness than zero-padded counterparts (0.41±0.05 vs. 0.49±0.06 respectively, P<0.001).
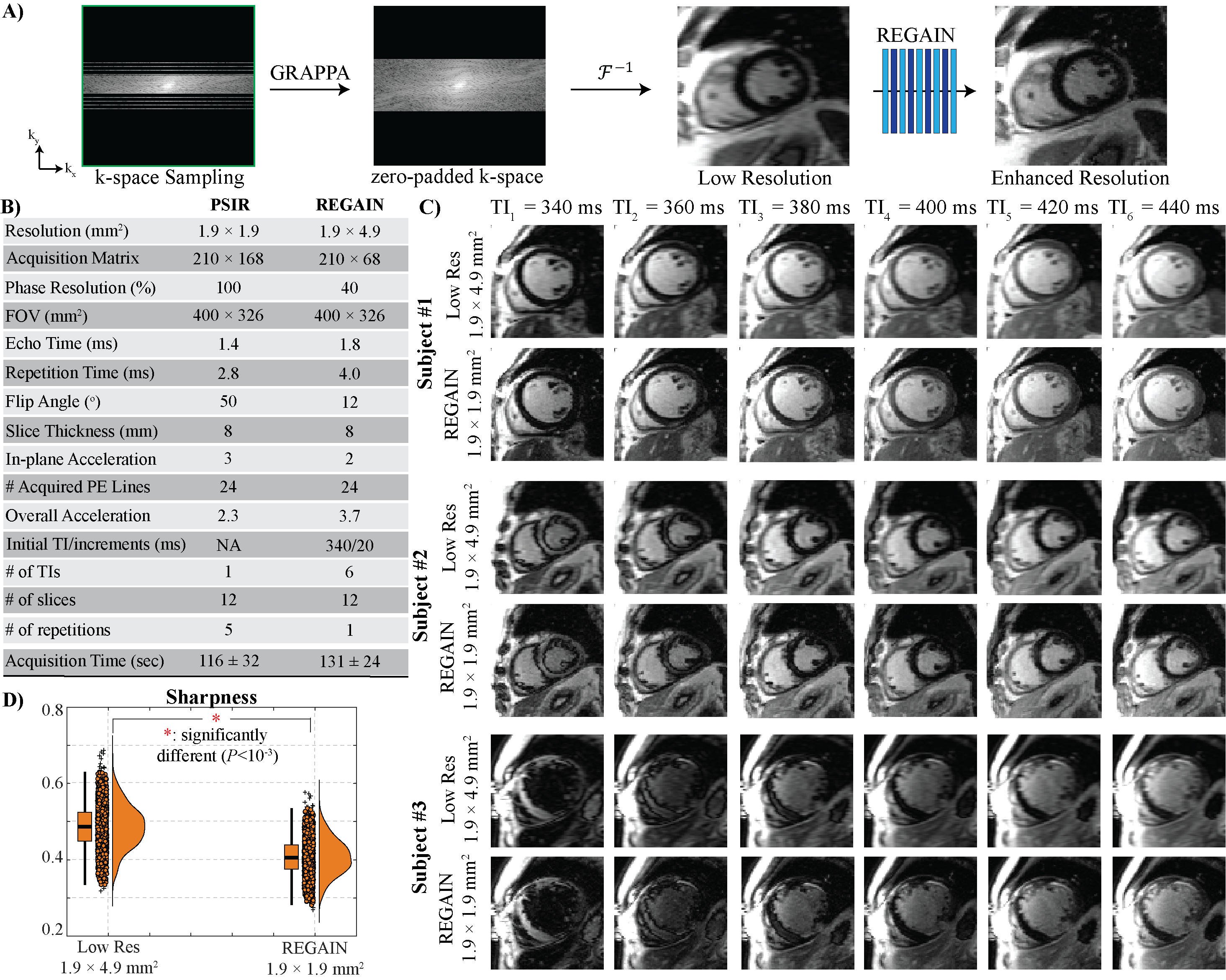
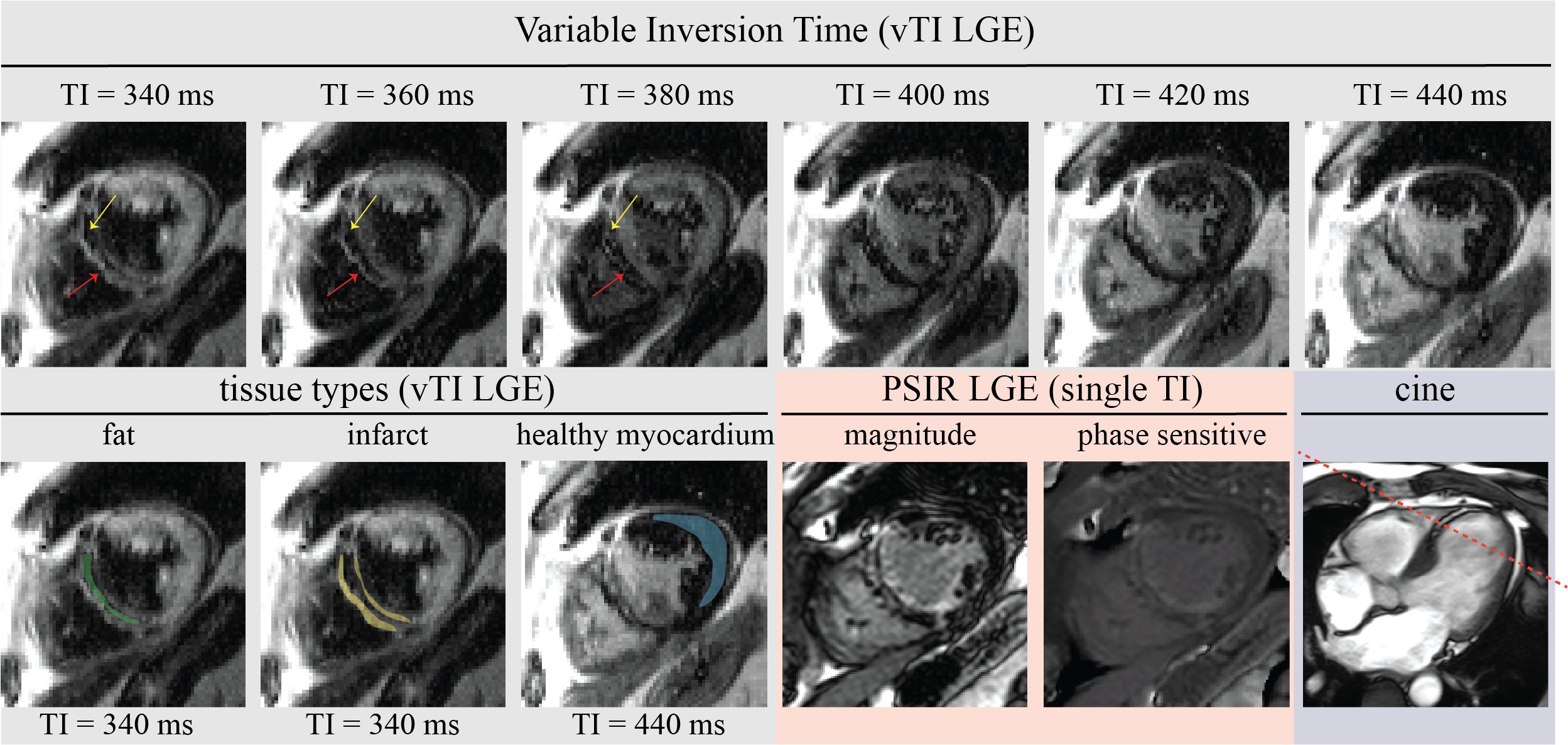
Figure 3: Representative free-breathing LGE images with variable inversion time vTI LGE (gray), PSIR (orange), and 4-chamber cine (purple) images are shown from a 78-year-old male patient with three-vessel coronary artery disease (percutaneous coronary intervention to the left anterior descending artery, right coronary artery, and ramus circumflex artery) and a history of myocardial infarction in 2019. The slice location is depicted on the cine images with the red line, which passes through the fat tissue. Six images across consecutive inversion times of a single slice are displayed. The infarct and fat tissues are shown by the yellow and red arrows respectively. The variable inversion time shows distinct signal recovery patterns, aiding in the differentiation of fat (green), infarct (yellow), and healthy myocardium (blue) signals shown in the tissue types section. LGE, consistent with the post-infarct transmural scar in the septum with fat infiltration, is visible in both vTI LGE and PSIR images. The proposed 2D LGE imaging with varying inversion times could facilitate the diagnostic process.
Background: Lipomatous metaplasia, a fatty infiltration of the myocardium, is associated with adverse outcomes in patients with healed myocardial infarction and has been associated with ventricular arrhythmia1. Accurate detection of fatty infiltration within the myocardium is challenging as fat appears bright in late gadolinium enhancement (LGE) images. Dixon-based methods can be used to image water and fat and have been combined with LGE imaging2,3. However, Dixon-based LGE method increases the scan time. In this study, we propose a free-breathing rapid 2D single-shot LGE sequence with varying inversion times (vTI LGE), allowing visualization of dynamic changes in signal intensity with different inversion times in the myocardium.
Methods:
Fig. 1A shows the proposed 2D vTI LGE imaging sequence. A single-shot LGE image is acquired in one heartbeat, followed by a dummy heartbeat. In the subsequent N heartbeat, LGE images are collected for the same slice by varying inversion time within a pre-specified range. The scan continues for other slices, covering the entire heart (Fig. 1B). To minimize the motion artifacts and blurring, k-space data corresponding to a low-resolution LGE image is acquired in each heartbeat. The resulting low-resolution images are then enhanced using a resolution enhancement generative adversarial inline neural network (REGAIN)4. The anisotropic low-resolution images (1.9×4.9 mm2) are first reconstructed using GRAPPA from 2-fold undersampled images, then zero-padded. Then, REGAIN enhances the resolution to 1.9×1.9 mm2 (Fig. 2A). REGAIN uses densely connected convolutions with residual blocks in its generator. Meanwhile, a relativistic generative adversarial network is used for the discriminator to generate realistic details. REGAIN is implemented inline via Siemens Framework for Image Reconstruction (FIRE)5. A pre-trained REGAIN model trained initially on ECG-gated segmented cine acquisitions was used without retraining for LGE data. To test the performance of the proposed method, we prospectively recruited adult subjects referred for a clinical CMR for viability imaging. Imaging was performed at 3T Siemens (MAGNETOM Vida). Low-resolution free-breathing acquisitions (Fig. 2B) with 210 × 68 matrix size were first GRAPPA reconstructed and then zero-filled to 210 × 168, representing the low-resolution images. REGAIN was applied to these low-resolution images.
Results: We recruited 30 subjects (56±15 years, 18 males) and performed full left ventricular coverage with six TIs in 131±24 seconds acquisition time. Fig. 2C shows example images from three subjects across six consecutive TIs (340ms with 20ms increments). REGAIN improved visual image sharpness (0.41±0.05 vs. 0.49±0.06, P< 0.001, Fig. 2D), significantly reducing blurriness compared to the low-resolution images. Fig. 3 shows images from vTI LGE, PSIR and cine of a patient with a healed infarct with significant fatty infiltration, showing different signal recovery patterns in fat, scar, and healthy myocardial areas, which are challenging to identify with standard phase-sensitive LGE sequences.
Conclusion:
A generative AI model enables free-breathing single-shot LGE imaging with variable inversion time, allowing visualization of signals with various TI in the same scan. The dynamic signal changes in the images allow simultaneous visualization of scar and fatty infiltration. Further validation and comparison with the Dixon-based technique multi-detector CT in patients with lipomatous metaplasia are needed.
Figure 1: Schematic of free-breathing single-shot LGE with varying inversion times. A) A reduced anisotropic k-space corresponding to a low-resolution image (1.9 × 4.9 mm2) is acquired with 2-fold GRAPPA at every other heartbeat. The changes in healthy myocardium, infarct, and fat signal intensity for different inversion times are visualized. B) This study acquires twelve slices and six TIs, starting from TI = 340 ms with 20 ms increments. The number of TIs and increment amount can be adjusted on the scanner console for finer steps or more contrasts. .png)
Figure 2: A) Initial GRAPPA reconstruction is followed by zero-padding and resolution enhancement using the generative adversarial inline neural network (REGAIN), which reconstructs images to a spatial resolution of 1.9 × 1.9 mm2 from 1.9 × 4.9 mm2. B) Imaging parameters of PSIR and REGAIN acquisitions are given in the table. 3.7-fold accelerated LGE images were acquired with 2-fold GRAPPA at 40% phase resolution, corresponding to a 210 × 68 matrix size, which was then zero-padded to 210 × 168. C) Representative free-breathing LGE images before and after image enhancement for three subjects with no LGE across six consecutive TIs. REGAIN reconstruction visibly improved image quality. The three patients achieved ideal nulling at 360, 400, and 340 ms, respectively. The third patient has subendocardial scars. D) Quantitative analysis of image sharpness using a reference-free blur metric (0: sharpest image quality, 1: blurriest image quality) showed that REGAIN-processed LGE images had significantly higher sharpness than zero-padded counterparts (0.41±0.05 vs. 0.49±0.06 respectively, P<0.001).
Figure 3: Representative free-breathing LGE images with variable inversion time vTI LGE (gray), PSIR (orange), and 4-chamber cine (purple) images are shown from a 78-year-old male patient with three-vessel coronary artery disease (percutaneous coronary intervention to the left anterior descending artery, right coronary artery, and ramus circumflex artery) and a history of myocardial infarction in 2019. The slice location is depicted on the cine images with the red line, which passes through the fat tissue. Six images across consecutive inversion times of a single slice are displayed. The infarct and fat tissues are shown by the yellow and red arrows respectively. The variable inversion time shows distinct signal recovery patterns, aiding in the differentiation of fat (green), infarct (yellow), and healthy myocardium (blue) signals shown in the tissue types section. LGE, consistent with the post-infarct transmural scar in the septum with fat infiltration, is visible in both vTI LGE and PSIR images. The proposed 2D LGE imaging with varying inversion times could facilitate the diagnostic process. 

