Rapid Fire Abstracts
Implicit Neural Representation for Motion-Corrected Multi-Echo Stack-of-Spirals Cardiac Quantitative Susceptibility Mapping (RF_TH_157)
- JL
Jiahao Li, MSc
PhD Candidate
Weill Cornell Medicine - JL
Jiahao Li, MSc
PhD Candidate
Weill Cornell Medicine - CL
Chao Li, MSc
PhD Candidate
Weill Cornell Medicine - PV
Pablo Villar-Calle, MD
Instructor in Medicine
Weill Cornell Medicine - RZ
Robert S. Zhang, MD
Instructor in Medicine
Weill Cornell Medicine - LJ
Lily Jin, BSc
Research Coordinator
Weill Cornell Medicine - MR
Mahniz Reza, BA
Research Assistant
Weill Cornell Medicine - AD
Alexey V. Dimov, PhD
Assistant Professor of Biomedical Engineering
Weill Cornell Medicine - YW
Yi Wang, PhD
Professor
Weill Cornell Medical College 
Jiwon Kim, MD
Associate Professor of Medicine
Weill Cornell Medicine
Jonathan W. Weinsaft, MD
Chief of Cardiology, Professor of Medicine
Weill Cornell Medicine
Pascal Spincemaille, PhD
Associate Professor of Physics Research In Radiology
Weill Cornell Medicine
Presenting Author(s)
Primary Author(s)
Co-Author(s)
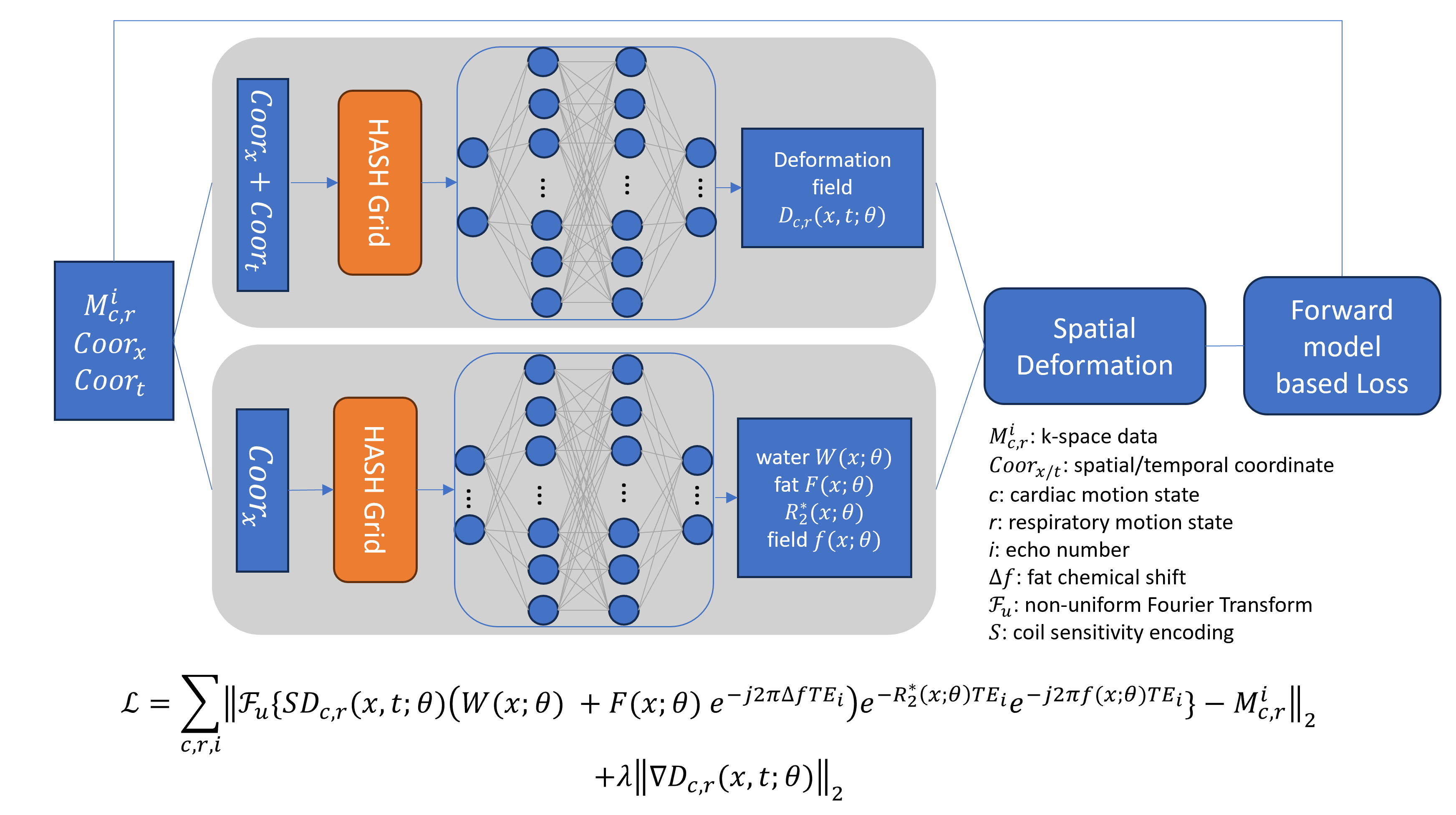
Figure 2. Motion-resolved cardiac MRI reconstruction of the first echo (TE=0.4msec) gradient echo images from implicit neural representation (INR). (A) Cardiac phases in (a-d) axial views, (e-h) coronal views and (i-l) sagittal views. The blue and orange arrows show the cardiac motion related epicardial fat and inferior vena cava (IVC) movement in axial views. Profiles of correspondent line in coronal (m) and sagittal (n) show the cardiac motion across the cardiac cycle. (B) Respiratory phases in (a-d) coronal view and (e-h) sagittal view. The blue dashed line shows the diaphragm position at the end-inspiration.
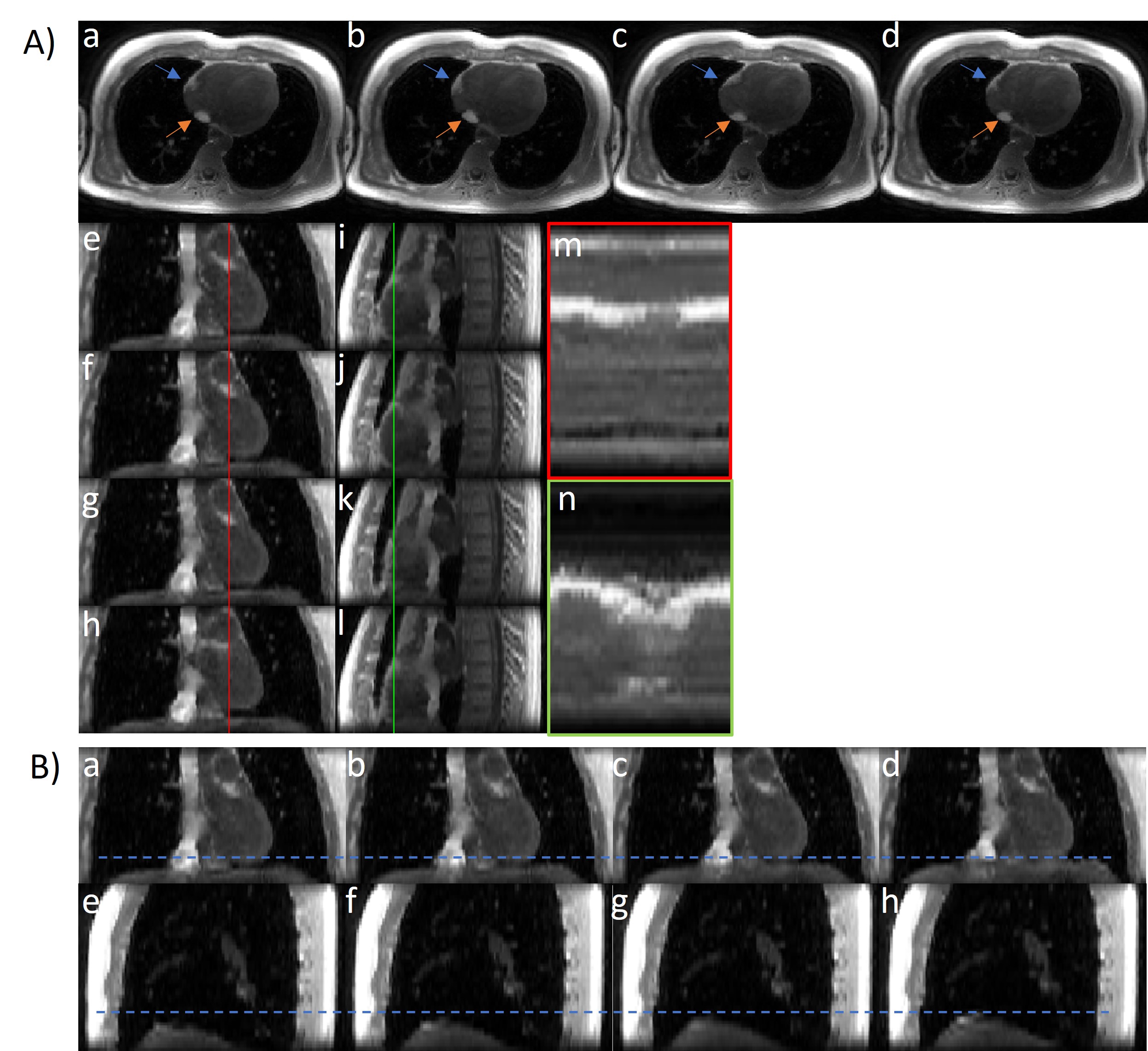
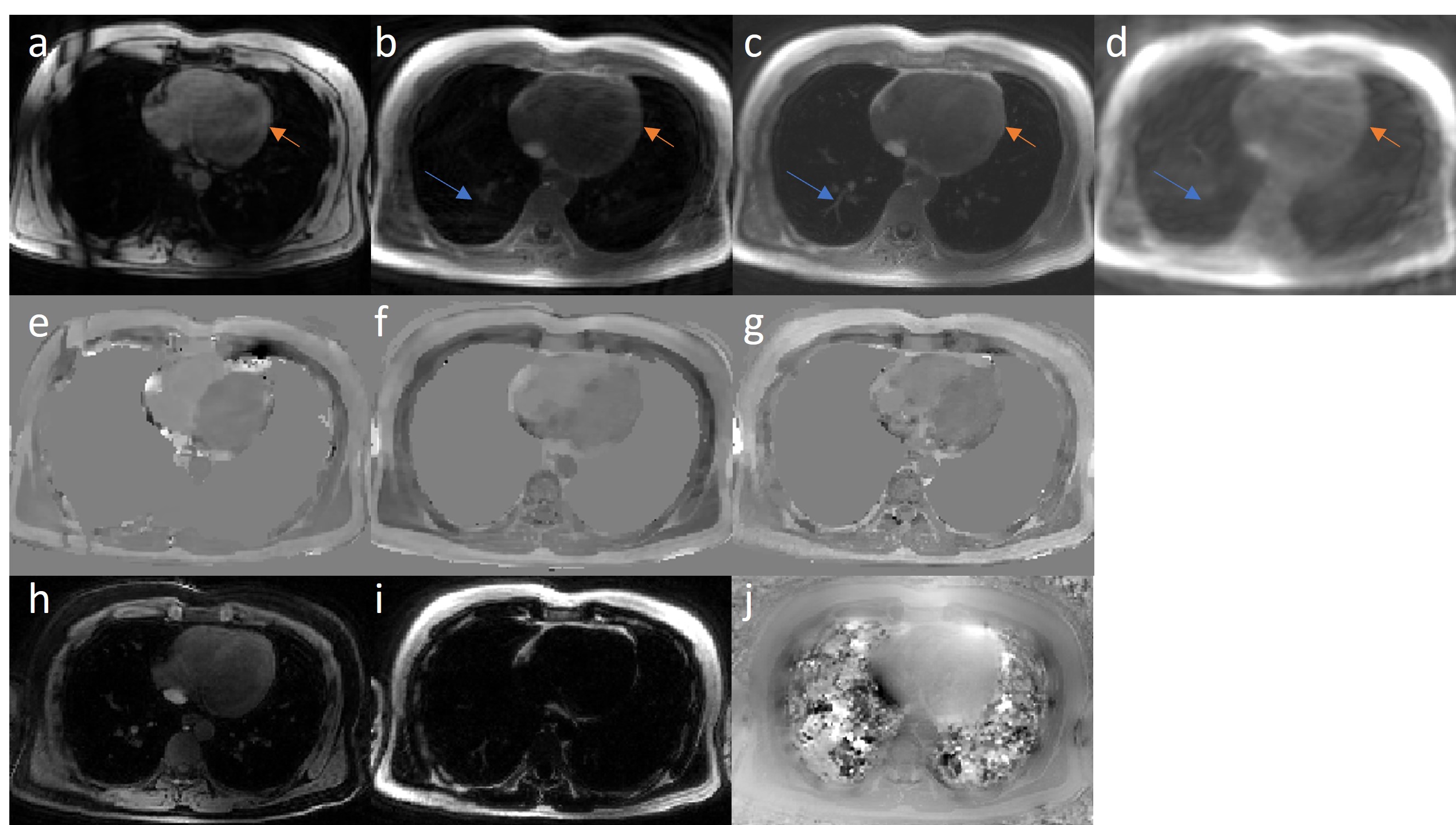
Figure 3. Motion corrected results in one representative case. The reconstructed first echo images are in the first row, (a) Cartesian reference (TE = 1.4msec), (b) motion average gridding, (c) implicit neural representation (INR) reconstruction and (d) XD-GRASP from proposed spiral acquisition (TE = 0.4msec). Arrows show INR reconstruction with fine details in the lung (blue) and free from artifacts (orange). XD-GRASP fails due to highly-undersampled acquisition. Second row shows correspondent TFI+0 QSM reconstruction, (e) Cartesian reference (∆SaO2, oxygenation difference between the right and left heart, 16.8%), (f) motion average (15.0%) and (g) INR (18.0%) from proposed spiral acquisition. INR QSM shows a shaper contrast in the heart compared to the motion average QSM. The last row shows the INR output, (h) water image, (i) fat image, and (j) field map, demonstrating the network ability to decompose the signal based on the physics-informed model and to generate parametric maps from spatial coordinates.
Background:
Cardiac quantitative susceptibility mapping (QSM)1,2 provides non-invasive imaging tool for differential blood oxygen saturation (∆SaO2). A non-gated stack-of-spirals sequence has been used for single breath-hold acquisition3. However, breath-holding can be challenging in many patients. Techniques have been developed for free-breathing cardiac MRI, including prospective navigator1 and XD-GRASP4. Deep learning has emerged to be a promising approach for motion correction5. In this study, we proposed a novel framework using retrospective navigator-based stack-of-spirals acquisition and implicit neural representation (INR) for motion corrected reconstruction and cardiac QSM.
Methods:
We used a stack-of-spirals multi-echo gradient echo sequence for free-breathing cardiac QSM. A superior-inferior navigator (SI-NAV) was acquired before each acquisition block. Imaging parameters were: axial FOV covering heart, each block with five randomly selected kz encodes and gold-angle rotated variable density spiral trajectory, 2880 leaves, readout bandwidth ±125kHz, flip angle 12º, 8 echoes, TE1/TR/ΔTE=0.4/14.0/1.1ms, resolution 1.8×1.8×5mm3, scan time 3m44s. For comparison, a free-breathing 1D diaphragmatic navigator ECG-gated 3D Cartesian sequence was acquired. K-space were sorted into 5D data with cardiac and respiratory motion dimensions estimated from SI-NAV6. Implicit neural representation (Figure 1) comprised of two multi-layer perceptrons (MLP): a first to reconstruct the motion deformation fields and a second to generate the static parametric maps (water, fat, R2* and field). A multi-resolution hash encoding was adopted for efficient input embedding with trainable parameters7. The network was trained in an instance-based self-supervised fashion with a loss function that uses a physics-informed motion-guided forward model, data fidelity and a TV regularization on the deformation. Total field inversion8 was applied on the output field to reconstruct QSM. For comparison, a motion-averaged image was also reconstructed. Motion extraction and QSM reconstruction was done in MATLAB 2023a; INR was implemented in PyTorch and trained on one NVIDIA RTX A6000 GPU.
Results:
Five healthy volunteers underwent free-breathing spiral and Cartesian navigator QSM. The INR reconstructed motion-resolved image from one representative case (Figure 2) indicated different cardiac and respiratory phases. INR showed motion-corrected results (Figure 3) free from artifacts. Estimated ∆SaO2 from INR-QSM was comparable with the reference Cartesian (14.0±3.5% vs 14.7±2.9%) as well as the motion average results (12.9±4.2%) among all the subjects.
Conclusion:
We demonstrate the feasibility of the proposed framework using implicit neural representation for motion-resolved multi-echo cardiac MRI reconstruction and motion-corrected cardiac QSM in this preliminary study.
Figure 1. Proposed implicit neural representation reconstruction pipeline. The above multi-layer perceptron (MLP) takes spatiotemporal coordinates to generate deformation field. The below MLP maps spatial coordinates to generate parametric maps. The loss function encodes the physics-informed forward signal model.
Figure 2. Motion-resolved cardiac MRI reconstruction of the first echo (TE=0.4msec) gradient echo images from implicit neural representation (INR). (A) Cardiac phases in (a-d) axial views, (e-h) coronal views and (i-l) sagittal views. The blue and orange arrows show the cardiac motion related epicardial fat and inferior vena cava (IVC) movement in axial views. Profiles of correspondent line in coronal (m) and sagittal (n) show the cardiac motion across the cardiac cycle. (B) Respiratory phases in (a-d) coronal view and (e-h) sagittal view. The blue dashed line shows the diaphragm position at the end-inspiration.
Figure 3. Motion corrected results in one representative case. The reconstructed first echo images are in the first row, (a) Cartesian reference (TE = 1.4msec), (b) motion average gridding, (c) implicit neural representation (INR) reconstruction and (d) XD-GRASP from proposed spiral acquisition (TE = 0.4msec). Arrows show INR reconstruction with fine details in the lung (blue) and free from artifacts (orange). XD-GRASP fails due to highly-undersampled acquisition. Second row shows correspondent TFI+0 QSM reconstruction, (e) Cartesian reference (∆SaO2, oxygenation difference between the right and left heart, 16.8%), (f) motion average (15.0%) and (g) INR (18.0%) from proposed spiral acquisition. INR QSM shows a shaper contrast in the heart compared to the motion average QSM. The last row shows the INR output, (h) water image, (i) fat image, and (j) field map, demonstrating the network ability to decompose the signal based on the physics-informed model and to generate parametric maps from spatial coordinates. 

