ISMRM - SCMR Workshop
Oral Abstract 2: Fully Automated Analysis of Cardiac Magnetic Resonance Diffusion Imaging
- KM
Kevin Moulin, PhD
Research Scientist
Boston Children's Hospital - KM
Kevin Moulin, PhD
Research Scientist
Boston Children's Hospital - TW
Tess E. Wallace, PhD
Senior Clinical MR Research Scientist
Siemens Medical Solutions USA, Inc. - JR
Jennifer Rodriguez
Clinical Trials Specialist
Beth Israel Deaconess Medical Center - JS
Jordan Street, N/A
Research Assistant
Beth Israel Deaconess Medical Center .jpg)
Magalie Viallon, PhD
Researcher
Univ Lyon, UJM-Saint-Etienne, INSA, CNRS UMR 5520, INSERM U1206, CREATIS, France
Pierre Croisille, MD, PhD
Professor
University Hospital Saint-Etienne, France.jpg)
Andy Powell, MD
Physician
Boston Children's Hospital
Boston Children's Hospital- RN
Reza Nezafat, PhD
Professor
Harvard Medical School
Presenting Author(s)
Primary Author(s)
Co-Author(s)
Cardiac diffusion tensor imaging (DTI) is a promising approach to explore the microstructural organization of myocardium without contrast. Quantitative parameters such as mean diffusivity (MD) and fraction of anisotropy (FA) can be directly calculated from the cardiac diffusion tensor, while absolute second eigen angle (|E2A|) and helix angle (HA) require tensor projection into the epicardial border1,2. These parameters are often computed offline after manually segmenting the left ventricle (LV), a time-consuming process. In this study, we propose a fully automated analysis platform for cardiac DTI. A self-configuring convolutional neural network (nnUnet)3 was trained and tested using a multi-center dataset for cardiac DTI analysis.
Methods:
The hyperparameters and layers of the nnUnet architecture were self-configured3. The input of the nnUnet was a single short-axis image, either diffusion-weighted image (DWI) or non-DWI. The network output was a mask representing the segmentation of the LV. We collected six cardiac DTI databases from two sites for training and testing of the network: Database A) reproducibility study on healthy (N=11), B) repeatability study on patients (N=17), C) HCM study (N=17), D) high-resolution study on healthy (N=64), E) multi-shot study on healthy (N=19), F) a DTI study on patients (N=19). All images were acquired using a spin-echo EPI research sequence with 1st and 2nd order motion compensation4,5 at 3T (Prisma and Vida, Siemens Healthineers, Forchheim, Germany). The images were acquired in mid-systole in navigated free-breathing conditions6 using various parameters (1-5 slices, b-values 0-350s/mm2, 6 -12 directions, resolution from 2.3x2.3x5 up to 1.6x1.6x8mm3 and TE from 55 up to 72ms). A total of 137 subjects were used corresponding to 1494 images for training and 374 images for testing. Manual epicardial and endocardial contours were drawn by an expert with 10 years of experience.
The DTI reconstruction pipeline is shown in Figure 1. MD and FA were calculated independently of the LV boundaries but were reported using both the expert and nnUnet segmentations. HA and |E2A| were calculated and reported using both segmentations. HA was reported transmurally in the Epi, Sub-Epi, Mid, Sub-Endo, and Endo regions. The difference between expert and nnUNET segmentation was evaluated using a dice score. Statistical differences between DTI parameters were evaluated using a paired T-test.
Results:
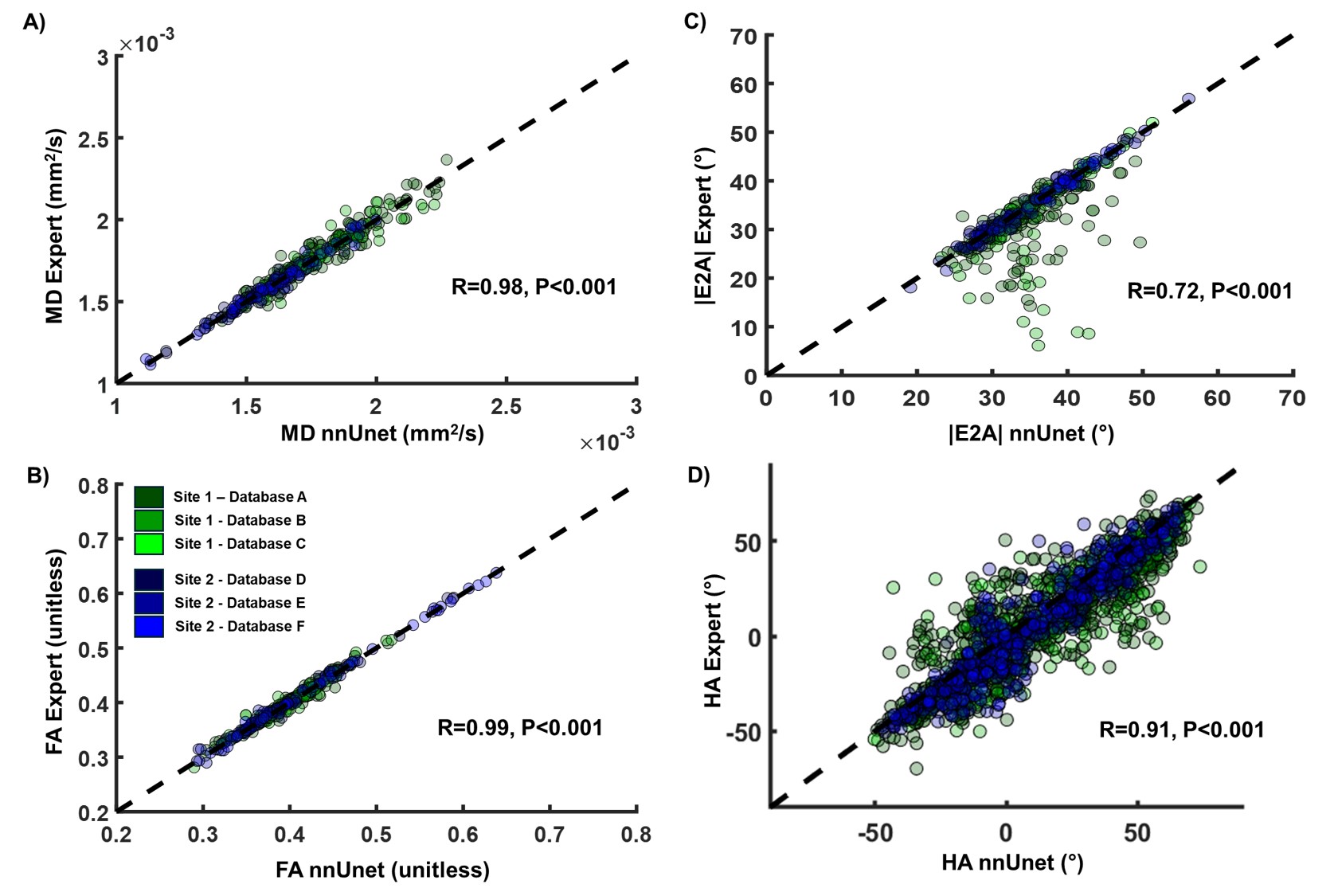
The segmentation was successful in 372 out of the 374 testing cases (99.47%). As shown in Figure 2, a Dice score of 0.93±0.04 was found between expert and nnUnet segmentations. Strong correlations were found for MD (r=0.99) and FA (r=0.98), while lower correlations were found for parameters requiring LV segmentation such as |E2A| (r=0.71), HA (r=0.91). Overall, no statistical differences were observed between expert and nnUnet-based parameters across all four DTI parameters (p< 0.01).
Conclusion:
The proposed open-access, fully automated cardiac DTI analysis platform provides an excellent correlation between automated and manual segmentation of cardiac DTI parameters.
Figure 1: Diffusion tensor imaging reconstruction steps. Diffusion-weighted images (DWI) and non-DWI images are fitted to a tensor model which can be used to calculate mean diffusivity (MD) and fraction of anisotropy (FA) maps. From the DWI image, expert segmentation and self-configuring convolutional neural network (nnUnet) segmentation of the left ventricle (LV) were performed to derive the epicardial and endocardial contours. These contours are used for tensor projection to estimate helix angle (HA) and absolute second eigen angle (|E2A|) maps. For MD and FA, expert and nnUnet LV segmentations were used only for reporting. For HA and |E2A|, both segmentations were used for reconstruction and reporting.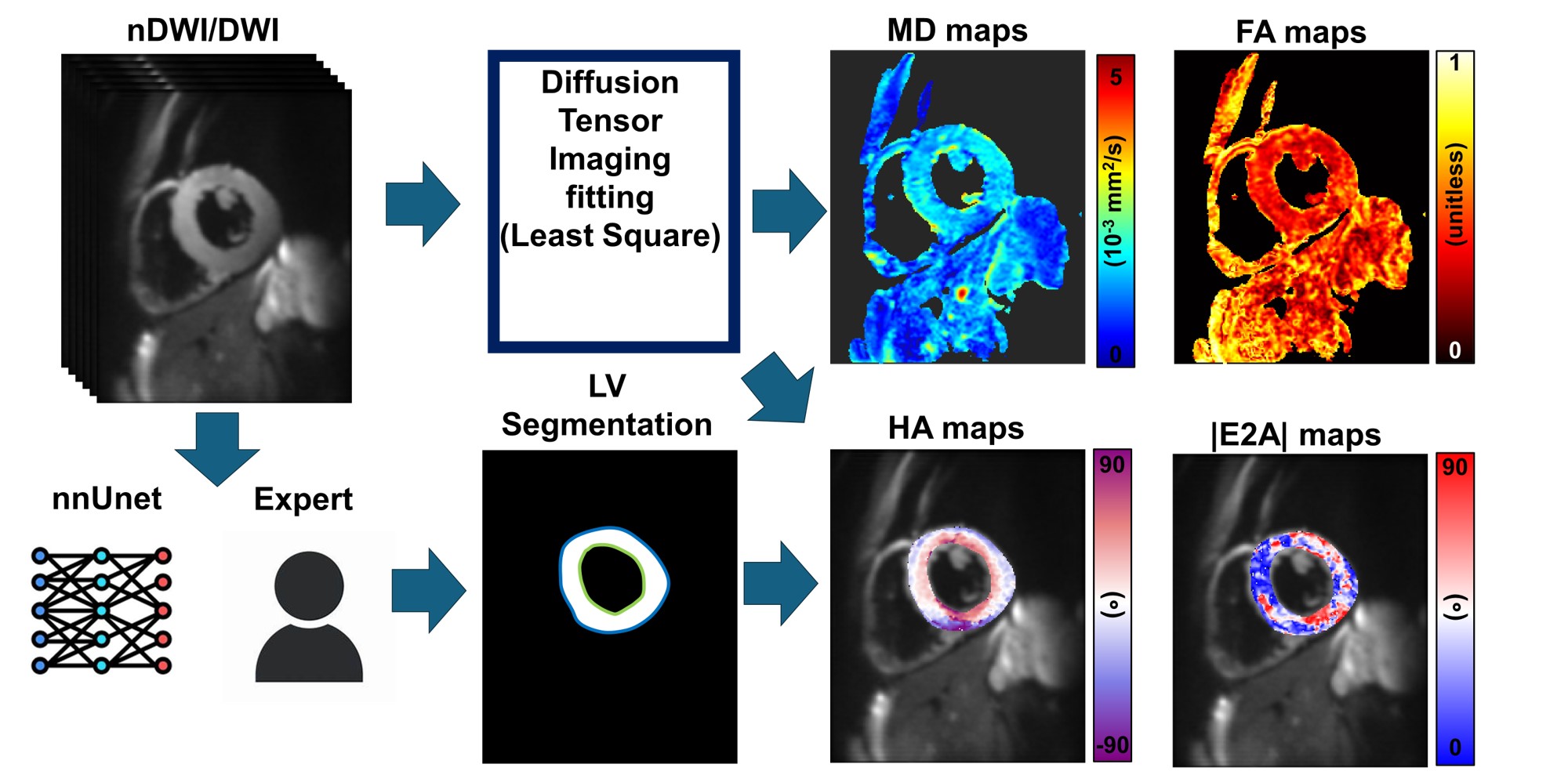
Figure 2: A) Dice score per testing cases across all databases between expert and self-configuring convolutional neural network (nnUnet) segmentations B) Example of the best and worst Dice score across databases.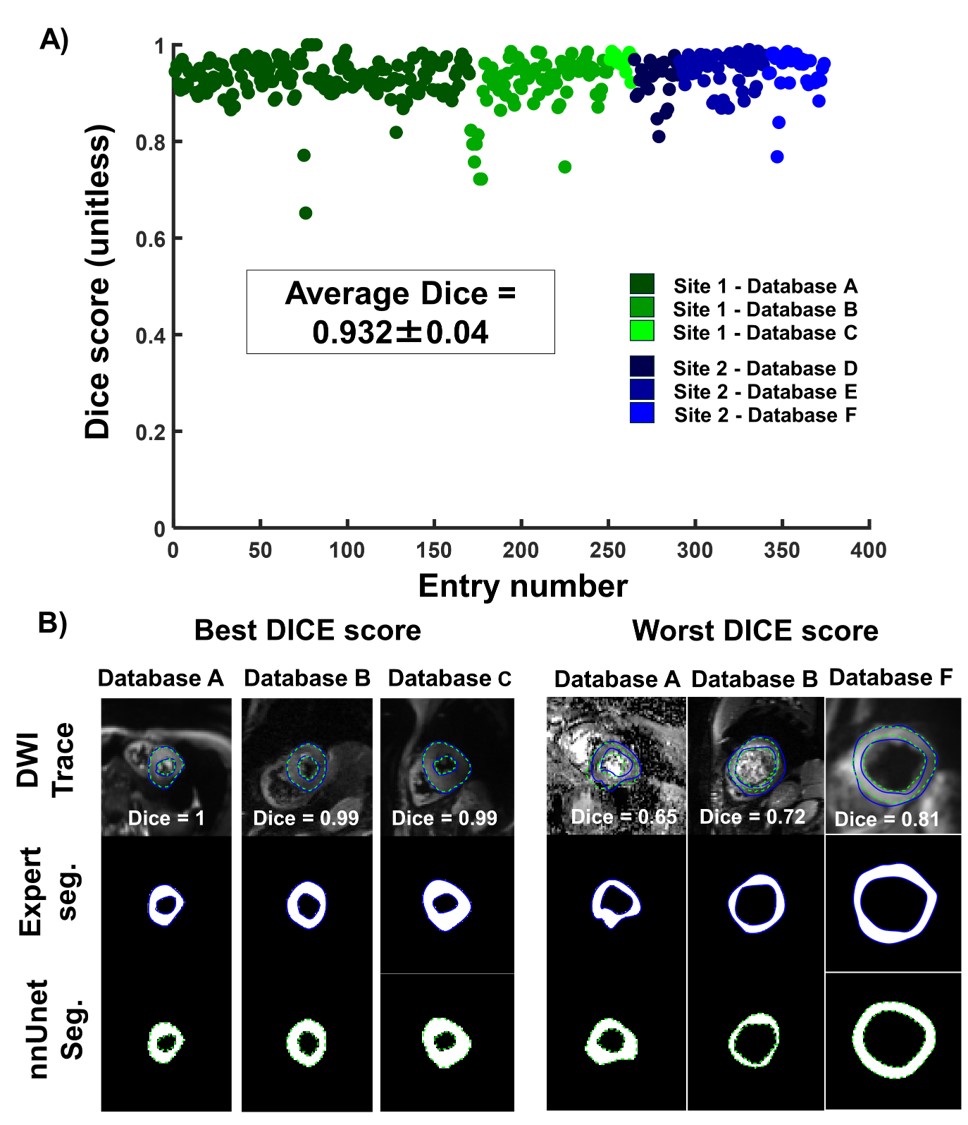
Figure 3: Correlation between expert and self-configuring convolutional neural network (nnUnet) reconstruction for A) mean diffusivity (MD), B) fraction of anisotropy (FA), absolute second eigen angle (|E2A|) and helix angle (HA) across the 5 transmural region across all datasets. For MD and FA, expert and nnUnet LV segmentations were used only for reporting. For HA and |E2A|, both segmentations were used for reconstruction and reporting. No statistical differences were observed between expert and nnUnet reconstruction for MD, FA, HA and |E2A|.