ISMRM - SCMR Workshop
Oral Abstract 2: Towards open-source spin-echo cardiac diffusion tensor imaging
- AH
Ariel J. Hannum, MSc
PhD Candidate
Stanford University - AH
Ariel J. Hannum, MSc
PhD Candidate
Stanford University - ML
Michael Loecher, PhD
Research Scientist
Stanford University - QC
Qingping Chen, MSc, BSc
PhD Student
University Medical Center Freiburg, Germany - EA
Eric Arbes, MSc, BSc
PhD Student
University Medical Center Freiburg, Germany - SL
Sebastian Littin
Head of Technology & Methods
University Medical Center Freiburg, Germany - KS
Kawin Setsompop, PhD
Associate Professor
Stanford University - MZ
Maxim Zaitsev
Head of Medical Physics
University Medical Center Freiburg, Germany 
Daniel B. Ennis, PhD
Professor
Stanford University
Presenting Author(s)
Primary Author(s)
Co-Author(s)
Clinical translation of cardiac diffusion tensor imaging (cDTI)1-5 is hindered by offline post-processing on separate workstations using custom software to perform motion correction, diffusion tensor fitting, and manually picking the center of the heart to generate helix angle (HA) maps. In this study, an inline cDTI post-processing package was implemented using the prototype Siemens framework for image reconstruction environments (FIRE)6 to achieve automated processing and on-scanner results visualization for cDTI.
Methods:
Fig1 shows the inline cDTI post-processing pipeline incorporating: 1) Motion correction using a pair-wise non-rigid image registration method7 directly on diffusion-weighted images (DWI). 2) Diffusion tensor fitting. 3) Automatic left ventricle (LV) detection using a U-Net pre-trained on images from 40 human subjects acquired with the same protocol using the MATLAB Deep Learning Toolbox. 4) HA map generation using myocardial center and diffusion tensor resulting from the steps above. This pipeline was implemented in a containerized MATLAB environment and runs on the local reconstruction computer (MARS) of the scanner. In addition to the original DWI, motion corrected images, mean diffusivity (MD) maps, fractional anisotropy (FA) maps, color FA maps, HA maps, and detected LV contours and myocardial center overlaid on DWI images were automatically returned and displayed on the scanner and available for exporting as DICOM.
Eleven healthy volunteers (age 23.8±3.4yo, 9 male) and 9 amyloid patients (age 72.6±5.9yo, 6 male) were scanned prospectively under an IRB approved protocol at 3T (MAGNETOM Cima.X, Siemens Healthineers, Forchheim, Germany). A research SE-EPI sequence with M2 compensated diffusion encoding8 and inner volume excitation was used to acquire images at end systole with ECG triggering under free-beathing. Acquisition parameters are: FOV=350mm×131mm, matrix=128×48, 5 slices, 8mm thick, TR=5 R-R, TE=59~67ms (PNS limited), 12 encoding directions, averages: 1 for b=50s/mm2, 8 for b=500s/mm2. Acquisition time was 8~10 min depending on the subject’s heart rate.
Results:
Inline post-processing for 5 slices (total 540 images) took 1 minute on scanner. LV border and myocardial center detection was successful in all volunteers. Representative results from one normal volunteer are shown in Fig2. Myocardial center detection was successful in all patients, although LV border detection was sub-optimal in 14 out of total 45 slices. On-scanner visualization of the diffusion tensor maps and native T1 and T2 maps in one amyloid patient is shown in Fig3. An ROI in septum shows elevated MD (1.89±0.05 µm2/ms), increased T1 (1504.93±43.61 ms) and T2 (45.33±2.14 ms), and decreased FA (0.16±0.02) compared with healthy subjects.
Conclusion:
Inline processing enables evaluation of diffusion parameters immediately on scanner or in PACS and enables wide-spread distribution and clinical evaluation of cDTI. Thus, this work implements a key step towards clinical use of cDTI.
Figure 1. Flow chart of the cDTI inline post-processing pipeline using FIRE. 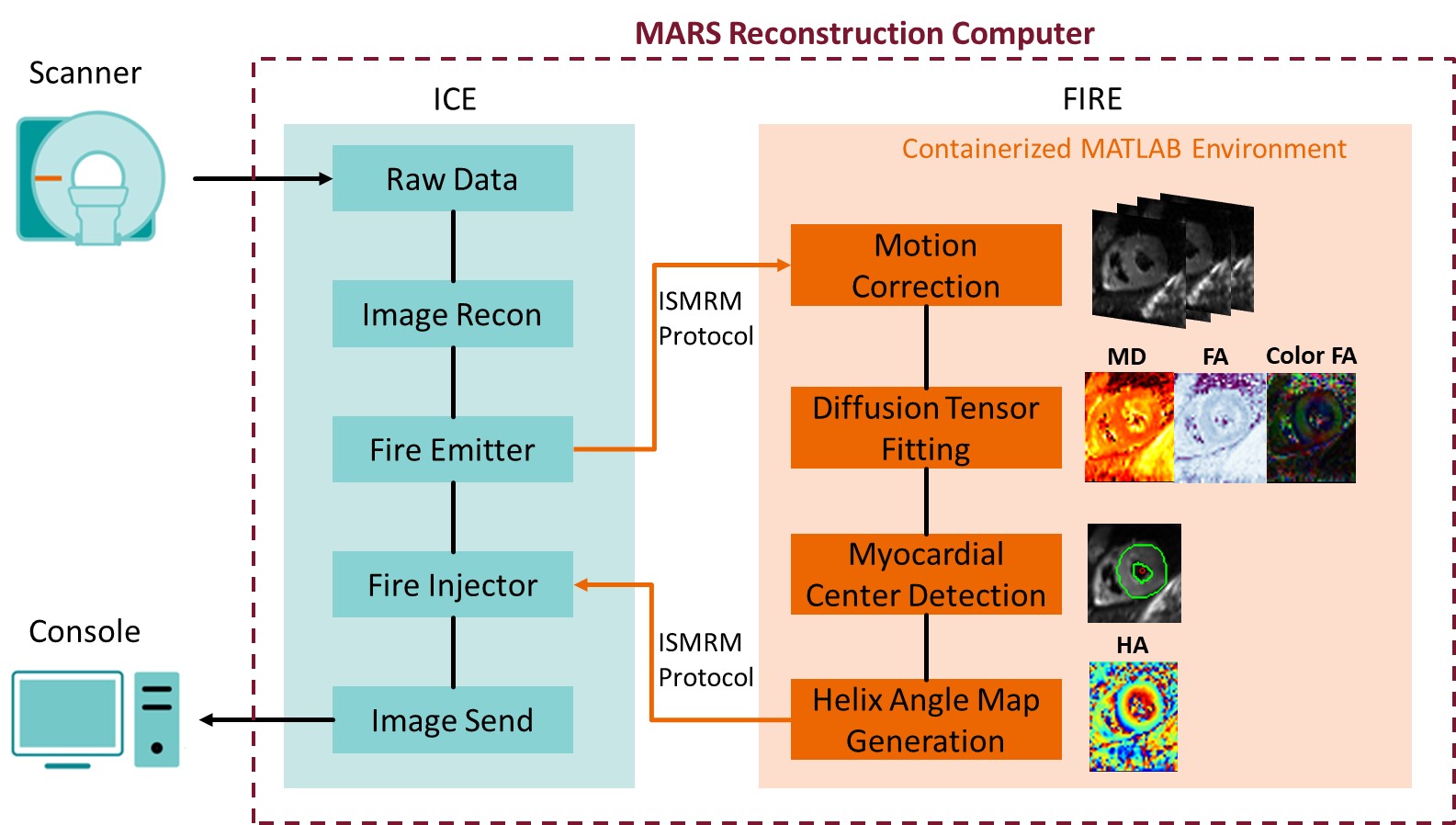
Figure 2. On-scanner visualization of the motion corrected image and diffusion tensor maps in a healthy volunteer. Apical slice MD 1.53±0.02 µm2/ms, FA 0.28±0.02; mid slice MD 1.47±0.05 µm2/ms, FA 0.29±0.02; basal slice MD 1.49±0.06 µm2/ms, FA 0.30±0.05 as measured in ROIs in septum on scanner..jpg)
Figure 3. On-scanner display of the motion corrected image and diffusion tensor maps along with native T1 map acquired using MOLLI and T2 map acquired using T2prep-FLASH in an amyloid patient. HA map was successfully generated regardless of sub-optimal LV detection. An ROI drawn in the septum shows decreased FA (0.16±0.02), elevated MD (1.89±0.05 µm2/ms), T1 (1504.93±43.61 ms), and T2 (45.33±2.14 ms) compared with healthy subjects. .jpg)

