Oral Abstract
AutoCMR: democratizing access to cardiac MRI with a push-button, 4D isotropic whole thoracic automated exam
- DK
Danielle Kara, PhD
Staff Scientist
Cleveland Clinic - DK
Danielle Kara, PhD
Staff Scientist
Cleveland Clinic - AD
Ashmita Deb, MSc
Research Data Scientist I
Cleveland Clinic - MR
Mary Robakowski, MSc
Graduate Student Researcher
Cleveland Clinic - HL
Hoa Le, MSc, BSc
PhD Candidate
Cardiovascular Innovation Research Center, Heart Vascular Thoracic Institute, Cleveland Clinic, Cleveland, OH, United States. Department of Biomedical Engineering, Case Western Reserve University & Cleveland Clinic, Cleveland, OH, United States. - MN
Makiya Nakashima, MSc
Research Data Scientist II
Cleveland Clinic - KB
Kashyap Bodi, MD
Research Fellow
Cleveland Clinic, Cleveland, OH, United States - YM
Yuncong Mao, BSc
Research Assistant
Cleveland Clinic - TR
Tassia Ribeiro Salles Moura, MSc
Graduate Researcher
Cleveland Clinic / Cleveland State University - HK
Heather Kohut, N/A
Research Technician
Cleveland Clinic - DM
Dingheng Mai
PhD Candidate
Cleveland Clinic - FK
Fayez Kanj, BSc
Medical Student
Cleveland Clinic - YP
Yea-Lyn Pak, BSc
Medical Student
Cleveland Clinic - AH
Angel Houston
MRI Tech
Cleveland Clinic - KK
Kathy Kohut, RT
MRI Technologist
Cleveland Clinic - MD
Mohsen Darayi, PhD
Staff Scientist
Cleveland Clinic - SC
Shi Chen, BSc
Research Coordinator
Cleveland Clinic - DW
Daniel Wee
Student Research Assistant
Cleveland Clinic - TG
Thomas Garrett, BSc
Research Assistant
Cleveland Clinic - WT
Wilson Tang, MD
Cardiologist
Cleveland Clinic - MB
Michael A. Bolen, MD
Staff Radiologist, Co-Section chief of CVI
Cleveland Clinic - DL
Daniel Lockwood, MD
Staff Physician
Cleveland Clinic - SJ
Stephen Jones, MD, PhD
Staff Physician
Cleveland Clinic - DG
Debkalpa Goswami, PhD
Director of Biomechanics
Cleveland Clinic 
Deborah Kwon, MD, FSCMR
Director of Cardiac MRI
Cleveland Clinic- DC
David Chen, PhD
Director of Artificial Intelligence
Cleveland Clinic 
Christopher Nguyen, PhD, FSCMR, FACC
Director, Cardiovascular Innovation Research Center
Cleveland Clinic
Presenting Author(s)
Primary Author(s)
Co-Author(s)
An extensive body of evidence supports cardiac magnetic resonance (CMR) as an integral modality for the diagnosis, prognosis, and treatment of cardiovascular disease1,2. Despite increasing demand, growth in CMR is slow3,4. Access to CMR is limited by the need for CMR training for technologists and physicians and time intensive analysis. Scheduling is difficult due to long, variable scan times. Conventional images are acquired in 2D views focused on the left ventricle (LV) with anisotropic resolution. Image quality is limited by breath-hold capability, reliable cardiac gating, and appropriate inversion time (TI) in delayed enhancement (DE) imaging.
Increasing adoption of CMR for cardiovascular care requires a paradigm shift in how CMR is performed. We propose AutoCMR: a push-button, free breathing, whole thoracic exam with 4D isotropic cine and DE imaging in 30 minutes and integrated analysis.
Methods:
80 patients were scanned with IRB approval using AutoCMR with Pusleq5,6 on a 3T Cima.X (Siemens Healthineers) (Fig. 1). A pre-scan was acquired in < 1min. Following injection of gadolinium contrast, 10 minutes of cine (GRE, TR/TE=4.0/2.9ms, FA=12°) then 15 minutes of DE (IRGRE, pulse-ox gated, TR/TE=3.7/2.6ms, FA=10°) data were collected with Gaussian random Cartesian sampling (307mm FOV, 1.6x1.6x1.6mm3). Total scan time was < 30 min. Cine data was binned into a mode respiratory phase and 30 cardiac frames with self-navigation7. DE data was respiratory motion and TI=[100:25:350]ms (100 ms window) binned. Images were reconstructed with ROVIr8, compressed sensing, and low rank denoising9. Auto-segmentation was performed with Swin-UNETR, trained on 48 manual segmentations. 2CH, 4CH, and SAX views were generated using segmentations.
Standard 2D cine (bSSFP, TR/TE=2.4/1.2ms, FA≤60°, 1.5mmx1.5mmx8mm) was acquired prior to AutoCMR during the same scanning session. LV metrics were compared using paired t-tests, Bland-Altman plots, and correlation.
Results:
AutoCMR cine and DE achieved whole thoracic coverage, enabling angiography and four-chamber functional assessment, with multiple TIs for tissue characterization (Fig. 2A). Automatically prescribed views demonstrated comparable image quality to standard cine and DE (Fig. 2B,D). AutoCMR DE with multiple TIs enables scar visualization with varying contrast.
LV volumes from standard and AutoCMR cine segmentations are correlated (LVEDV, LVESV: R2>0.8, Fig. 3). AutoCMR underestimated LVEDV (6.64mL) and overestimated LVESV (-2.99mL). AutoCMR LVEF and LVCO have 3.95% and 0.32L/min biases. LV blood pool was often overestimated in systole, particularly with hypertrophy, contributing to LVESV and LVEF bias. Although correlated (R2=0.77), AutoCMR images have limited myocardium-fat delineation, contributing to LVM overestimation.
Conclusion:
AutoCMR is a free-breathing, 30 minute protocol with 4D isotropic, whole thoracic coverage enabling cardiac morphology, function, and tissue characterization at the push of a button. By simplifying the cardiac MRI exam and its analysis, AutoCMR has the potential to democratize CMR access.
Figure 1: A. Comparison of Conventional CMR and AutoCMR, which features reduced technologist interactions, dependable and reduced scan duration, no breath holds, 3D isotropic cine and delayed enhancement imaging with multiple inversion times (TI), and automated whole heart segmentation, metric extraction, and view finding. B. UI Mouse tracking for conventional CMR and AutoCMR exams. While conventional CMR requires 96 ± 56 interactions from the MRI technologist, AutoCMR requires only 1 click to start the scan and a few additional interactions for the contrast injection. C. Conventional CMR scan duration distribution for 50,000 patients, with average scan duration of 50 minutes and a large variation in scan duration across patients. D. Comparison of normal vectors to traditional short axis (SAX) viewing planes in 80 patients showing general agreement between views automatically generated with AutoCMR and prescribed by a cardiac MRI technologist.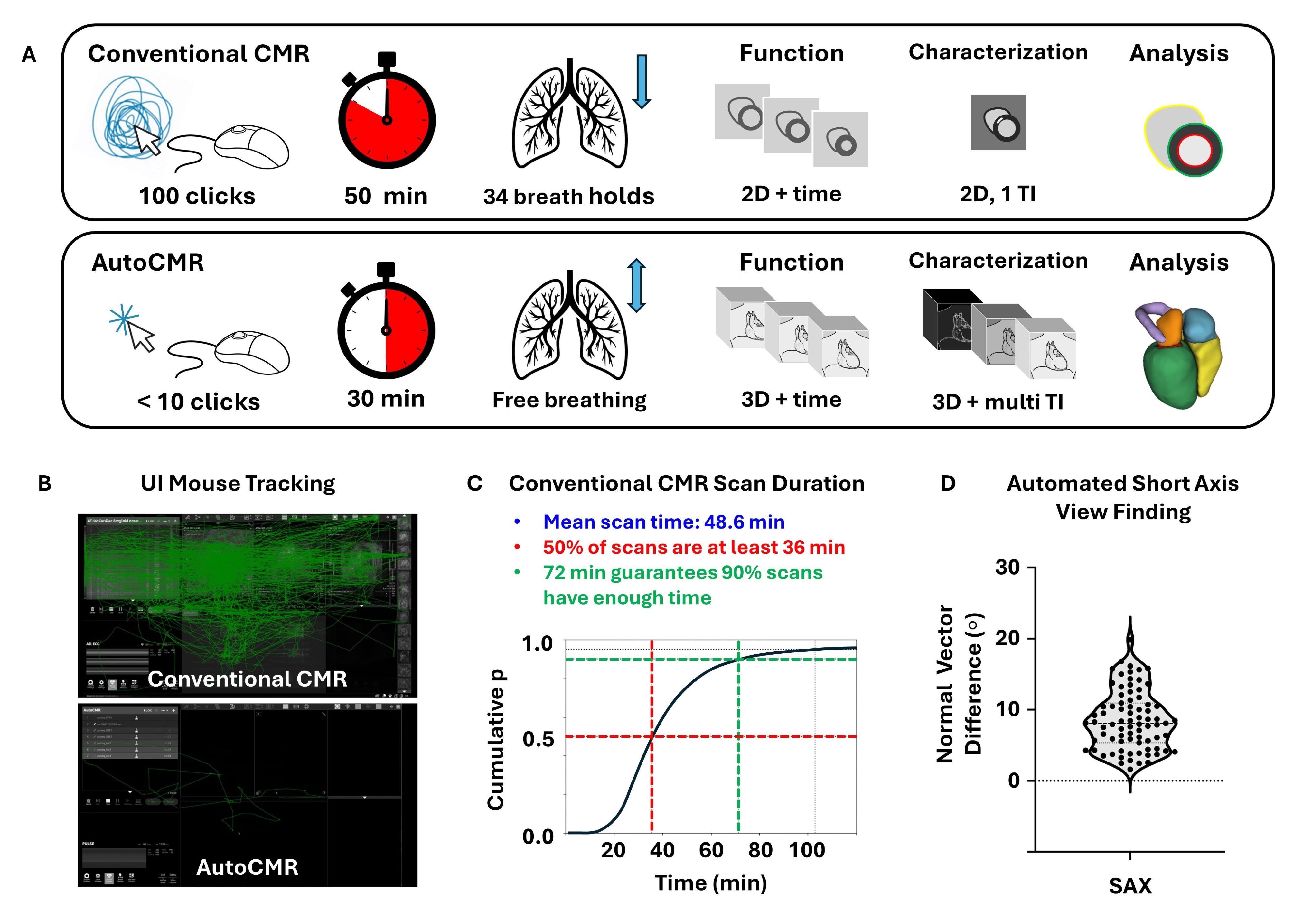
Figure 2: A. AutoCMR provides 3D isotropic cine data with high spatial and temporal resolution. Automatic segmentations of the cine volumes enable automated image rotation into traditional views (2CH, 4CH, SAX) and volumetric reporting. While image quality and scar visualization in DE imaging for conventional CMR exams depends on the appropriate choice of TI, AutoCMR generates 3D isotropic DE images at multiple inversion times (TI), enabling dark-blood, gray-blood, and bright-blood scar visualization. AutoCMR cine images (B), cine segmentations (C), and DE images (D, E) from representative volunteers are qualitatively comparable to those obtained in a conventional, clinical exam. Late gadolinium enhancement (yellow arrows) is visible in DE images in 2CH and SAX views. (E) AutoCMR DE images with multiple TI times provide varying contrast options for LGE visualization. 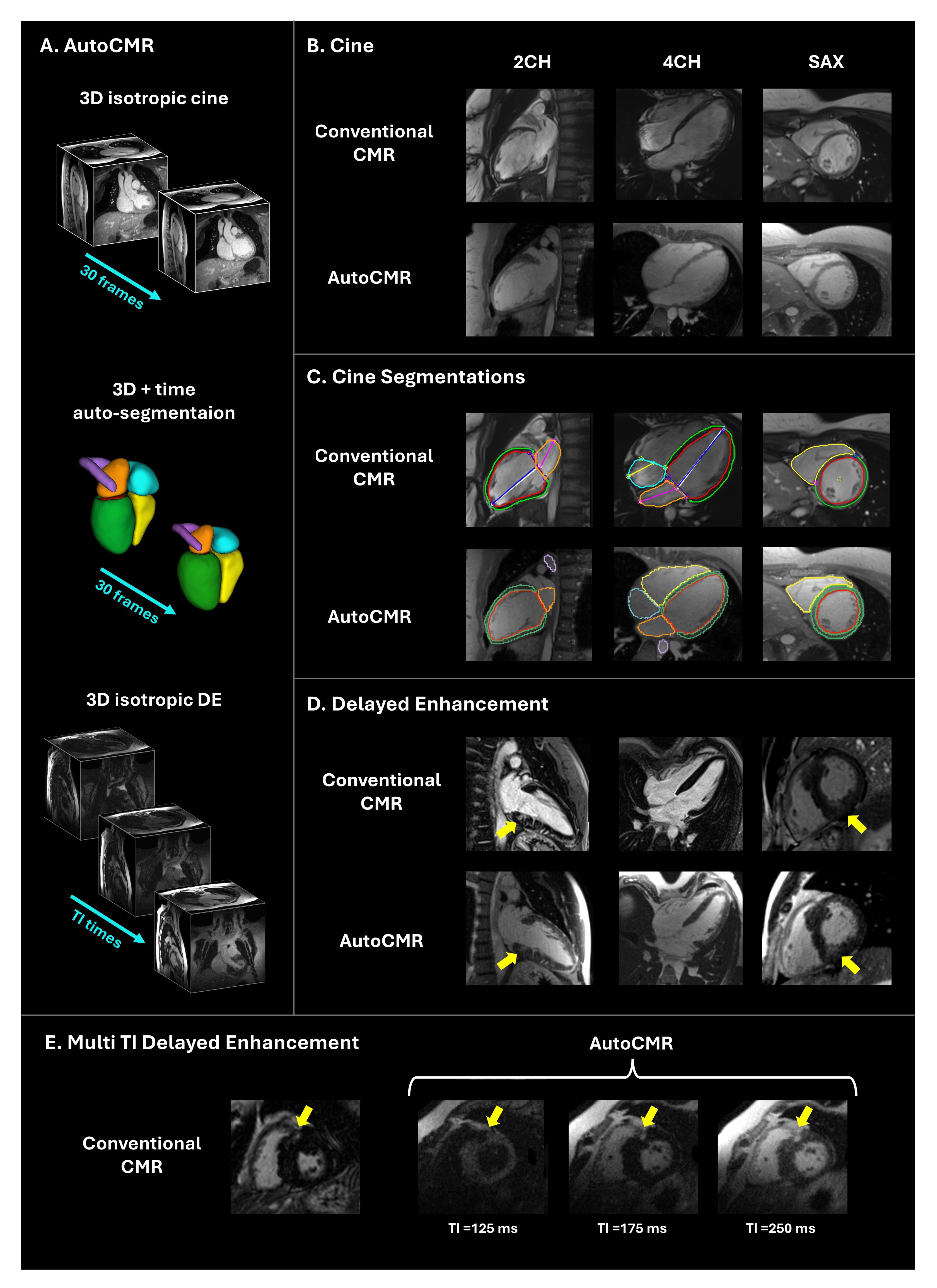
Figure 3: Left ventricular metrics (LVEDV: left ventricular end diastolic volume, LVESV: left ventricular end systolic volume, LVEF: left ventricular ejection fraction, LVCO: left ventricular cardiac output, LVM: left ventricular mass) in 80 patients from 2D conventional cine imaging and 3D AutoCMR cine are compared using paired t-tests (left), Bland-Altman analysis (middle), and correlation plots (right). .jpg)

