Early Career
Exercise CMR and proteomic characterization of HFpEF across the spectrum of LV ejection fraction
- RX
Roshan Xavier, MD, MA
Clinical Research Fellow
Oxford Centre for Clinical Magnetic Resonance Research, United Kingdom - RX
Roshan Xavier, MD, MA
Clinical Research Fellow
Oxford Centre for Clinical Magnetic Resonance Research, United Kingdom - SN
Sher May Ng, MD, MA
Clinical Research Fellow
Oxford Centre for Clinical Magnetic Resonance Research, United Kingdom - JP
Jiliu Pan, MD, MA
Clinical Research Fellow
Oxford Centre for Clinical Magnetic Resonance Research, United Kingdom - PA
Per M. Arvidsson, MD, PhD
Postdoctoral Researcher
University of Oxford, United Kingdom - JM
Jack JJJ Miller, PhD
Associate Professor
Aarhus University, United Kingdom - FM
Ferenc E. Mózes, PhD
Postdoctoral Research Assistant
Oxford Centre for Clinical Magnetic Resonance Research, United Kingdom - LV
Ladislav Valkovič, PhD
Associate Professor
Oxford Centre for Clinical Magnetic Resonance Research, United Kingdom - JR
Jenny J. Rayner, MD, PhD
Consultant Cardiologist
Oxford Centre for Clinical Magnetic Resonance Research, United Kingdom 
Will D. Watson, BSc
Clinical Lecturer
University of Cambridge, United Kingdom- MR
Marzia Rigolli, MD, PhD
Director, Global Drug Development
Bristol Myers Squibb - MF
Matthew Fronheiser, PhD
Cardiovascular imaging lead
Bristol Myers Squibb 
Stefan Neubauer, MD, PhD
Professor
Oxford Centre for Clinical Magnetic Resonance Research, United Kingdom- OR
Oliver J. Rider, MD, PhD
Professor
Oxford Centre for Clinical Magnetic Resonance Research, United Kingdom 
Andrew JM Lewis, DPhil FRCP
Cardiologist and Principal Investigator
Radcliffe Department of Medicine, United Kingdom
Presenting Author(s)
Primary Author(s)
Co-Author(s)
Heterogeneity within heart failure with preserved ejection fraction (HFpEF) is poorly understood and hinders the development of new therapies for this condition. Despite comparable symptom burden, clinical trials suggest differential treatment responses between HFpEF patients with resting LV ejection fraction above or below 60%. We used ergometer stress CMR coupled with in-magnet proteomic profiling to precisely characterize whole-heart exercise responses of patients with HFpEF across the spectrum of LV ejection fraction.
Methods:
HFpEF Characterization Study: 64 patients with HFpEF (along with 16 controls) underwent 3T CMR imaging at rest and during supine exercise at 35W (Siemens Magnetom Prisma). Free-breathing, real-time cine imaging of the whole heart was performed at rest and following 5 minutes of exercise. Pulmonary congestion was quantified using a novel MR proton density sequence. Plasma samples were obtained during the MRI scan for aptamer-based proteomic profiling (7000 circulating proteins). Exercise echocardiography, lung ultrasound, and 6-minute walk test were also performed in all participants. HFpEF patients were dichotomized into two groups according to resting LV ejection fraction by CMR (HFpEF>60% versus HFpEF< 60%).
Metabolic Intervention Study: Based on the CMR and proteomic results from the HFpEF Characterization Study, we subsequently proceeded to a second study to test the effects of metabolic modulation in HFpEF. A further 24 HFpEF patients were randomized to receive either glucose, lipid or oral ketone administration to modulate cardiac energy metabolism with CMR again used to quantify changes in cardiac function at rest and during exercise.
Results:
HFpEF Characterization Study: When compared to HFpEF< 60% (n=18), patients with HFpEF>60% (n=46) had smaller ventricles at rest (LVEDVi 65 ± 15 vs 94 ± 17ml/m2, p< 0.001), blunted systolic augmentation (LVEF +3 ± 5 vs +5 ± 4%, p=0.03), lower stress cardiac index (4.6 ± 1.1 vs 5.2 ± 1.3 L/min/m2, p=0.048), lower pulmonary congestion during exercise (∆proton density -0.5 [-3.7, 2.7] vs +3.5% [1.8, 7.7], p=0.02, ∆Kerley B lines 1.6 [0, 2.8] vs 3.3 [1, 4.8], p=0.02), and shorter 6-minute walk distance (349 ± 95 vs 401 ± 76m, p=0.04). Proteomic profiling of samples obtained during the exercise CMR revealed that changes in protein sets for lipid metabolism were linked with impairment of LV contractile reserve in HFpEF, thus suggesting a possible target for metabolic intervention.
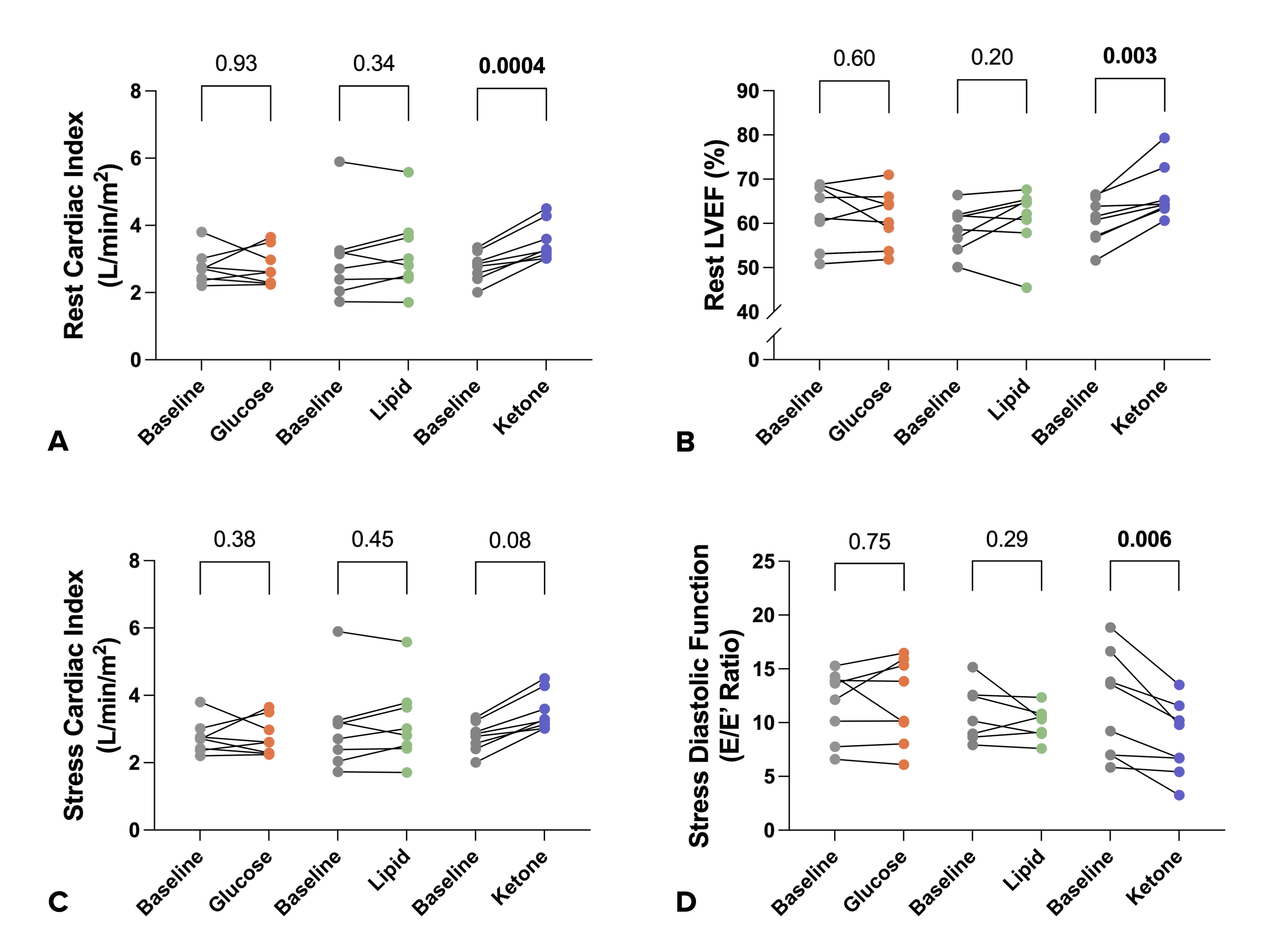
Metabolic Intervention Study: In HFpEF patients, neither glucose nor lipid administration significantly altered cardiac function at rest or during exercise. However, ketone administration augmented cardiac output by CMR at rest (2.8 ± 0.4 vs 3.5 ± 0.6 L/min/m2, p< 0.001) and stress (4.6 ± 0.8 vs 5.5 ± 0.9 L/min/m2, p=0.08) and this was corroborated by improved stress diastolic function on exercise echocardiography (E/e’ 12 ± 5 vs 8 ± 3, p=0.006). Combined exercise CMR and proteomic profiling in HFpEF demonstrated marked differences in cardiac exercise responses across the spectrum of LV ejection fraction, and these were linked with altered circulating metabolic protein sets. Metabolic modulation in HFpEF with oral ketone improved cardiac output at rest and exercise and rescued stress diastolic function. Precise rest and exercise CMR measures of whole heart function hold promise for future HFpEF medicines development trials, including novel cardiac metabolic modulators.
Conclusion:
HFpEF Characterization Study: Pathway enrichment analysis on stress proteomics revealed abnormal protein sets for lipid metabolism in both HFpEF groups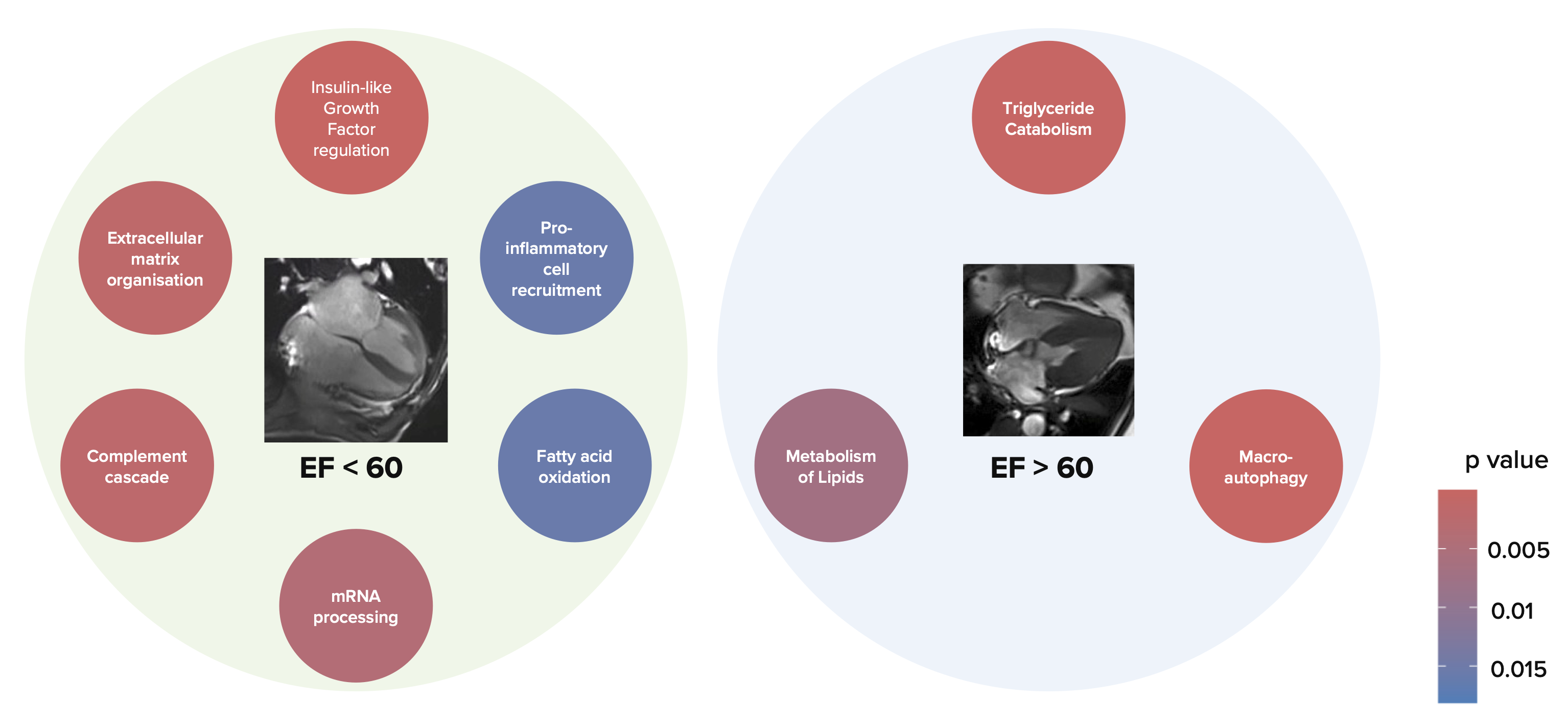
Metabolic Intervention Study: Ketone ester administration resulted in increased cardiac output at rest (A) and stress (C) as well as LV ejection fraction at rest (B). In addition, it also improved diastolic function at stress (D)