Early Career
Fractional anisotropy as a diffusion CMR derived metric for early remodeling in hypertrophic cardiomyopathy
- DK
Danielle Kara, PhD
Staff Scientist
Cleveland Clinic - OL
Oumaima Laghzali, MSc
PhD Student
Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany. Charité—Universitätsmedizin Berlin, Germany. DZHK (German Centre for Cardiovascular Research), partner site Berlin, Berlin, Germany, Germany - SC
Shi Chen, BSc
Research Coordinator
Cleveland Clinic - DK
Danielle Kara, PhD
Staff Scientist
Cleveland Clinic - SS
Shahriar Shalikar, MSc
PhD Student
Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany., Germany - MR
Mara-Camelia Rusu, PhD
Senior researcher
Electron Microscopy Technology Platform, Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany., Germany - LC
Lucie Carrier, PhD
Prof.Dr.
Department of Experimental Pharmacology and Toxicology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany. DZHK (German Centre for Cardiovascular Research), partner site Hamburg/Kiel/Lübeck, Hamburg, Germany., Germany 
Thoralf Niendorf, PhD
Prof.Dr.
Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany. Experimental and Clinical Research Center, Charite Medical Faculty and the Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany. DZHK (German Centre for Cardiovascular Research), partner site Berlin, Berlin, Germany., Germany
Christopher Nguyen, PhD, FSCMR, FACC
Director, Cardiovascular Innovation Research Center
Cleveland Clinic- MK
Min-Chi Ku, PhD
Senior researcher
Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany. DZHK (German Centre for Cardiovascular Research), partner site Berlin, Berlin, Germany, Germany
Presenting Author(s)
Primary Author(s)
Co-Author(s)
Diffusion tensor imaging (DTI) is a powerful noninvasive technique for assessing myocardial microstructure using metrics such as fractional anisotropy (FA), mean diffusivity (MD), and helix angle (HA) [1]. These markers are promising for detecting early myocardial changes similar to those observed in hypertrophic cardiomyopathy (HCM) [2, 3], a disease that is typically diagnosed at a later stage by the presence of hypertrophy. This study aims to evaluate the effectiveness of cardiac DTI-derived metrics in identifying early HCM-associated changes in both human patients and a mouse model.
Methods:
We performed CMR scans on a cohort of 10 patients with HCM and 10 healthy controls using a 3T MRI equipped with a strong gradient system (MAGNETOM Cima.X, Siemens Healthineers AG, Erlangen, Germany). The DTI sequence parameters were as follows: 2D radiofrequency zoomed diffusion-prepared spin echo, field of view = 350 mm, matrix = 128 x 48, 5 slices, 8-mm slice thickness, TR = 5 R-R intervals, 1 b0 acquisition, 12 diffusion directions at b = 500 s/mm2, and 8 averages. In a separate animal study, 6 wild-type (WT) and 6 homozygous 7-8-week-old Mybpc3-knock-in (KI) mice were scanned in vivo using cine-CMR to assess cardiac function and strain. Their hearts were subsequently scanned ex vivo on a 9.4T system (Biospec 94/20, Bruker Biospin, Germany) using a 3D-EPI sequence: TE = 26.4 ms, TR = 2000 ms, resolution = 125 x 125 x 306 µm, and b-value = 3000 mm². For both cohorts, global metrics including MD, FA and HA were calculated over the entire LV. Post scans, the left ventricular free wall of all mice hearts was analyzed using scanning electron microscopy (SEM) for ultrastructural assessment, including Z-disc offset measurement to evaluate misalignment near intercalated discs.
Results:
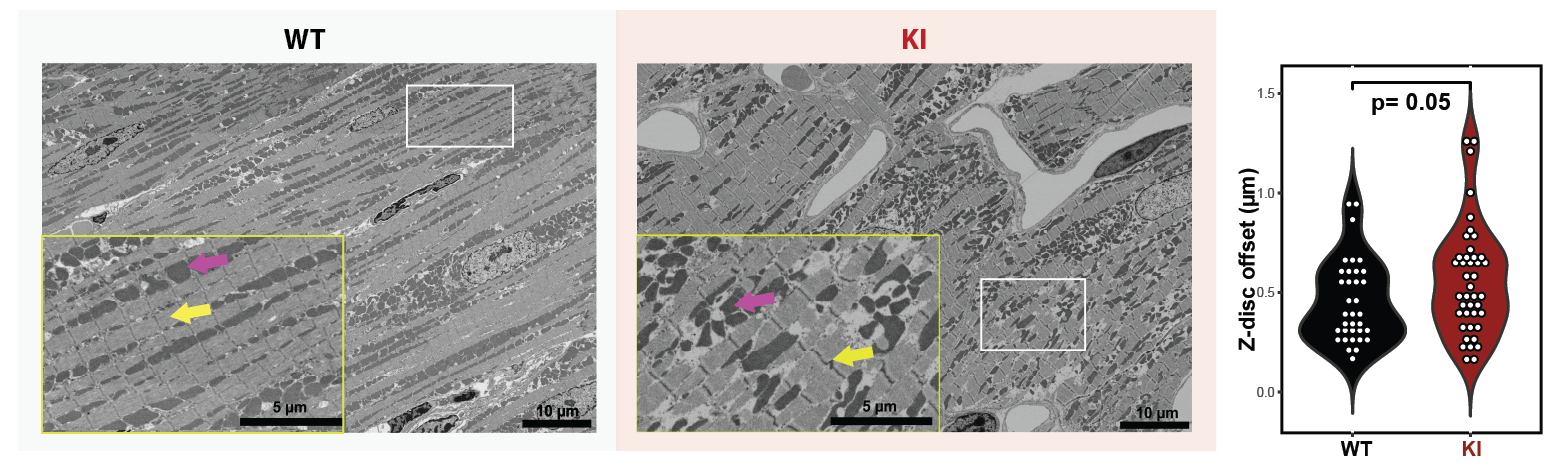
In the human cohort, only DTI metrics showed significant differences while cardiac function and strain remained unchanged (Figure 1). FA was lower in the HCM group than in controls (FAHCM = 0.29 ± 0.03 vs. FAHealthy = 0.34 ± 0.02, p= 0.002; Figure 1), suggesting a certain degree of altered myocardial microstructure. In the mouse cohort, HCM mice exhibited altered cardiac function and strain, which was corroborated by changes in DTI metrics (FAKI= 0.25 ± 0.02 vs. FAWT = 0.32 ± 0.01, p=0.0001; HAKI = -0.83 ± 0.09 vs. HAWT= -1.01 ± 0.08, p=0.007; Figure 2). SEM further confirmed disrupted myofibrillar alignment in KI mice, aligning with the DTI findings (Figure 3).
Conclusion:
FA could uniquely detect early regional disarray of cardiomyocytes, highlighting its value in early HCM detection across species, even without changes in other DTI parameters like HA or MD. SEM data confirmed ultrastructural disarray, supporting the FA findings. Further studies are needed to explore myocyte structure, extracellular matrix changes, and their link to functional impairment to fully understand FA's role in HCM pathology.
Figure 1: Assessment of cardiac function and diffusion tensor imaging (DTI) reveals changes in anisotropy in the absence of systolic dysfunction. A) In the patient cohort, the left ventricular thickness (LVT) was higher, while left ventricular ejection fraction (LVEF) remained unaffected. Additionally, left ventricular strain analysis, including global circumferential strain (GCS), global longitudinal strain (GLS), and global radial strain (GRS) did not differ between the groups. B) DTI analysis shows lower fractional anisotropy (FA) values in the hypertrophic cardiomyopathy (HCM) group than in healthy controls. Mean diffusivity (MD) and helix angle (HA) did not differ significantly between the groups. Data are expressed as mean ± SD. Statistical analysis was performed using unpaired Student’s t-test. Significance levels are indicated as p-values and colored in red when the significance is reached p<0.05..png)
Figure 2: Investigating cardiac function and diffusion tensor imaging (DTI) in a mouse cohort reveals cardiac dysfunction and notable changes in anisotropy and helix angle. A) Mybpc3-Knock-in (KI) mice displayed higher left ventricular thickness (LVT) and lower systolic function, indicated by lower left ventricular ejection fraction (LVEF) than wild-type (WT) mice. Analysis of muscle strain, including global circumferential strain (GCS), global longitudinal strain (GLS), and global radial strain (GRS), showed significant differences between KI and wild-type (WT) mice. B) DTI analysis demonstrated lower fractional anisotropy (FA) and higher helix angle (HA) values in KI mice than in WT controls. Mean diffusivity (MD) did not differ significantly between the groups. Data are expressed as mean ± SD. Statistical analysis was performed using unpaired Student’s t-test. Significance levels are indicated as p-values and colored in red when the significance is reached p<0.05..png)
Figure 3: Representative SEM micrographs of WT and KI mouse cardiomyocytes. WT mice exhibit typical sarcomeric band distribution (yellow arrows) and mitochondrial arrangement (magenta arrow). KI mice show regions of subtle sarcomeric disarray (yellow inset), though not uniformly present. Violin plot of Z-disc offset near intercalated discs reveals no statistically significant difference between groups (3 WT male, 3 WT female, 3 KI male and 3 KI female). Nevertheless the distribution of offsets is suggestive of early-stage ultrastructural changes in KI mice. The subtle nature of these alterations may be due to the young age of the mice, with potential for more pronounced myofibrillar disarray to develop over time. While not visible in SEM, further investigation using alternative techniques may provide additional insights into HCM-related structural changes in this model.