Rapid Fire Abstracts
Inline Strain Analysis for DENSE CMR (RF_TH_226)

Sona Ghadimi, PhD
research scientist
University of Virginia
Sona Ghadimi, PhD
research scientist
University of Virginia- JE
John T. Echols
PhD Candidate
University of Virginia - KC
Kelvin Chow, PhD
MR Collaboration Scientist
Siemens Healthcare Ltd., Canada, Canada - TW
Tess E. Wallace, PhD
Senior Clinical MR Research Scientist
Siemens Medical Solutions USA, Inc. - CD
Cristiane C. De Carvalho Singulane, MD
Postdoctoral Researcher
University of Virginia Health System 
Amit R. Patel, MD
Professor of Medicine
Division of Cardiology, University of Virginia Health System, Charlottesville, Virginia, USA.
Frederick H. Epstein, PhD
Professor
University of Virginia
University of Virginia
Presenting Author(s)
Primary Author(s)
Co-Author(s)
.png)
Fig. 2: Screenshot of the inline strain analysis results displayed on the scanner console. Panels A and B show the acquired phase-wrapped images encoded for displacement in the x and y directions, respectively. Panels C and D display the unwrapped phases of the segmented myocardium, while Panel E shows the circumferential strain cine map. Panel F includes whole-slice and segmental strain-time curves, and end-systolic circumferential strain quantifications. All analysis outputs are displayed within 15 seconds after DENSE acquisition directly on the console.
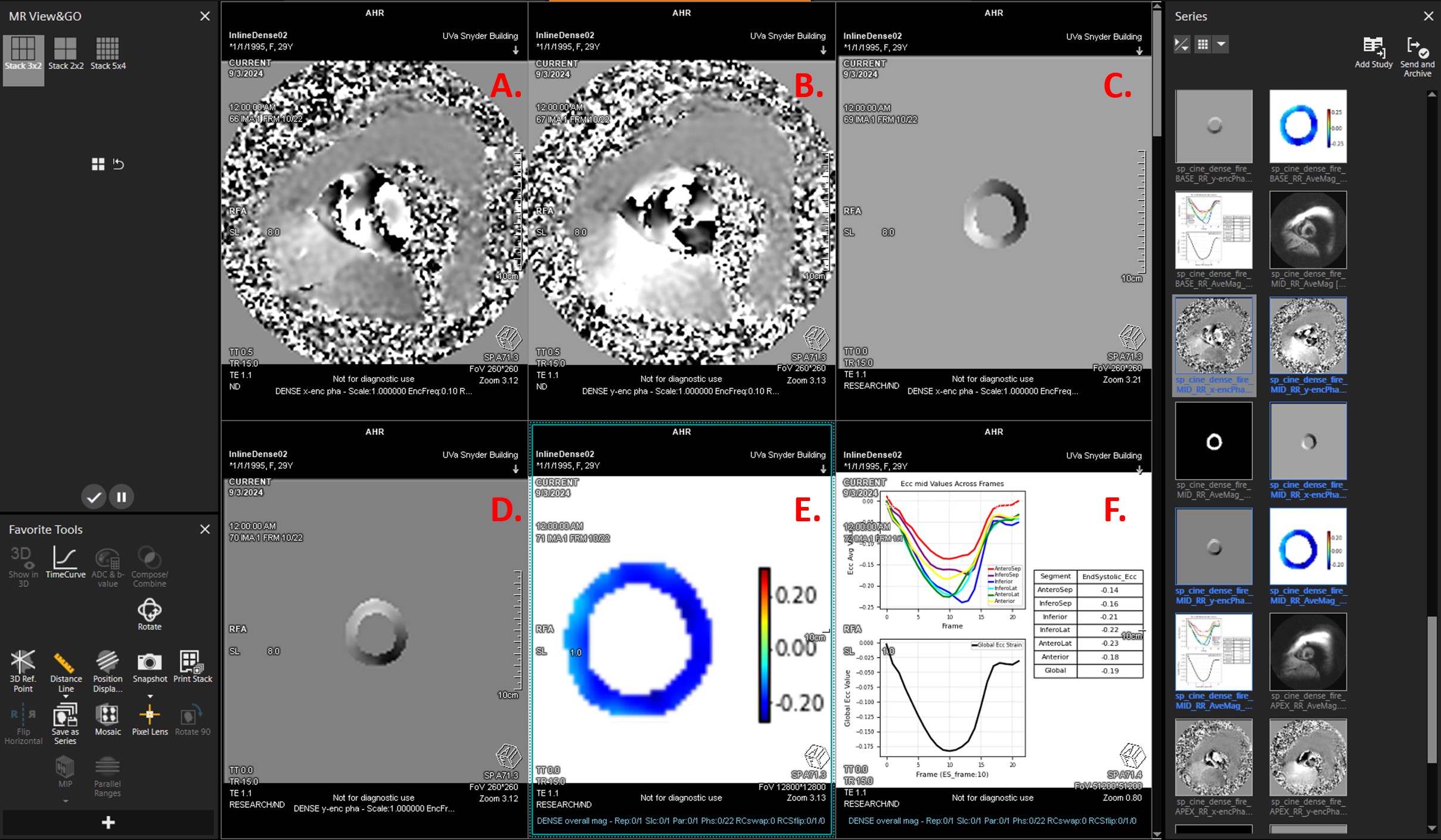
Fig. 3: Correlation and Bland-Altman plots for whole-slice and segmental circumferential strain (Ecc), along with a bull’s eye plot showing the coefficient of variation (CV) for segmental Ecc comparing inline and offline strain.
.jpg)
Background:
Displacement encoding with stimulated echoes (DENSE) CMR is accurate and reproducible for whole-slice and segmental strain measurements and it is well-suited for fully automatic and rapid strain analysis using deep learning methods1-3. Strain analysis in CMR has traditionally been performed offline, but this can be time consuming and the results are not available to provide guidance during scanning. We propose that inline strain analysis of DENSE images is feasible and that implementation directly on the scanner would eliminate the need for separate post-processing, deliver immediate results, and facilitate a more efficient clinical workflow. For applications like strain that are not yet well established as a routine part of the CMR workflow, inline analysis could lower the barrier to clinical adoption.
Methods:
We developed a fully automated framework for inline strain analysis, integrated directly on a 3T scanner (MAGNETOM Prisma, Siemens Healthineers, Germany) using the Siemens Framework for Image Reconstruction Environment (FIRE) research prototype4. DENSE images were reconstructed in the Siemens Image Calculation Environment (ICE), and the reconstructed images were analyzed in a FIRE containerized Python environment (Fig. 1). The analysis system performs 3D (2D+time) segmentation, phase unwrapping, Lagrangian displacement estimation, circumferential strain calculation, visualization of cine strain maps, and the generation of whole-slice and segmental strain-time curves with end-systolic strain quantification. A 3D-UNet with a Visual Geometry Group encoder was used for segmentation and phase unwrapping, while the RV-LV insertion point was automatically extracted using a 2D-UNet model for segmental strain reporting, and the Lagrangian displacement was computed using the regularized spatiotemporal least squares method5. Four healthy volunteers underwent DENSE imaging with inline analysis providing real-time output DICOM images containing the myocardial mask, unwrapped phase images, multiphasic circumferential strain maps, whole-slice and segmental strain-time curves, and end-systolic circumferential strain quantification in under 15 seconds (Fig. 2). Eleven previously acquired DENSE raw datasets were retrospectively analyzed to validate the accuracy of the inline method compared to existing offline user-interactive DENSE analysis6. Comparisons of end-systolic whole-slice and segmental circumferential strain between inline and offline reference methods were performed using correlation and Bland-Altman analyses and by computing the coefficient of variation (CV).
Results:
The inline analysis produced circumferential strain maps and whole-slice and segmental strain-time curves, displayed on the scanner within 15 seconds of the end of image acquisition. Comparison of inline and offline analyses revealed strong agreements for both whole slice (r=0.96) and segmental circumferential (Ecc) strains (r=0.89) (Fig. 3A-D). The CV for the whole-slice inline vs offline analysis was 1.84% and 6.29 ± 2.73% for the segmental analysis, demonstrating good reproducibility (Fig. 3E).
Conclusion:
Fully automated inline DENSE strain analysis demonstrated excellent agreement with the reference offline method, offering an efficient, real-time solution for myocardial strain assessment with high potential to improve the clinical workflow. Future work will extend the validation to patient populations and will investigate automated methods for quality control.
Fig.1: Schematic overview of the FIRE framework for strain analysis. After image reconstruction by DENSE-ICE, the reconstructed images are processed in a FIRE containerized Python environment to perform 3D (2D+time) segmentation, phase unwrapping, Lagrangian displacement estimation, circumferential strain calculation, and visualization of cine strain maps, along with segmental strain-time curves and end-systolic strain quantification. Results are returned to the scanner console and viewable immediately..png)
Fig. 2: Screenshot of the inline strain analysis results displayed on the scanner console. Panels A and B show the acquired phase-wrapped images encoded for displacement in the x and y directions, respectively. Panels C and D display the unwrapped phases of the segmented myocardium, while Panel E shows the circumferential strain cine map. Panel F includes whole-slice and segmental strain-time curves, and end-systolic circumferential strain quantifications. All analysis outputs are displayed within 15 seconds after DENSE acquisition directly on the console.
Fig. 3: Correlation and Bland-Altman plots for whole-slice and segmental circumferential strain (Ecc), along with a bull’s eye plot showing the coefficient of variation (CV) for segmental Ecc comparing inline and offline strain..jpg)

