Oral Case
A Case of Dual Diagnosis - Anderson-Fabry Disease with Hypertrophic Cardiomyopathy: Diagnostic Insights and Imaging Findings
- TL
Thanatcha Lertnimittham, MD
Cardiac MRI fellowship
Ramathibodi Hospital, Mahidol University, Thailand - TL
Thanatcha Lertnimittham, MD
Cardiac MRI fellowship
Ramathibodi Hospital, Mahidol University, Thailand - sk
surachai kongrat, MD
Dr.
Ramathibodi Hospital, Mahidol University, Thailand
Presenting Author(s)
Primary Author(s)
Co-Author(s)
A 55-year-old Thai male with a family history of Anderson-Fabry disease (AFD) was referred to the cardiology department for cardiac screening. His mother, who exhibited clinical heart failure, revealed a mutation in the GLA gene, and she was subsequently managed with enzyme replacement therapy.
In this patient, genetic testing indicated decreased activity of the alpha-galactosidase enzyme (< 0.8 μmol/L/hr) and identified a likely pathogenic mutation in the GLA gene. He also had the pathogenic mutation of ALPK3 gene, which associated with dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM). Although, he remained asymptomatic. He reported no chest pain or dyspnea on exertion and had no history of stroke or neurological symptoms. His vital signs were within normal limit.
To further assess the cardiac involvement of AFD, the patient was scheduled for echocardiography and cardiac magnetic resonance imaging (CMR).
Diagnostic Techniques and Their Most Important Findings:
His echocardiography revealed normal left ventricular systolic function (LVEF 63% by the biplane method) with increased LV wall thickness, which maximal thickness of 24 mm in the mid-inferoseptal segment. The study also identified a mid-cavitary gradient of 20 mmHg at rest, increasing to 60 mmHg during the Valsalva maneuver, without evidence of LV outflow tract obstruction (Figure 1).
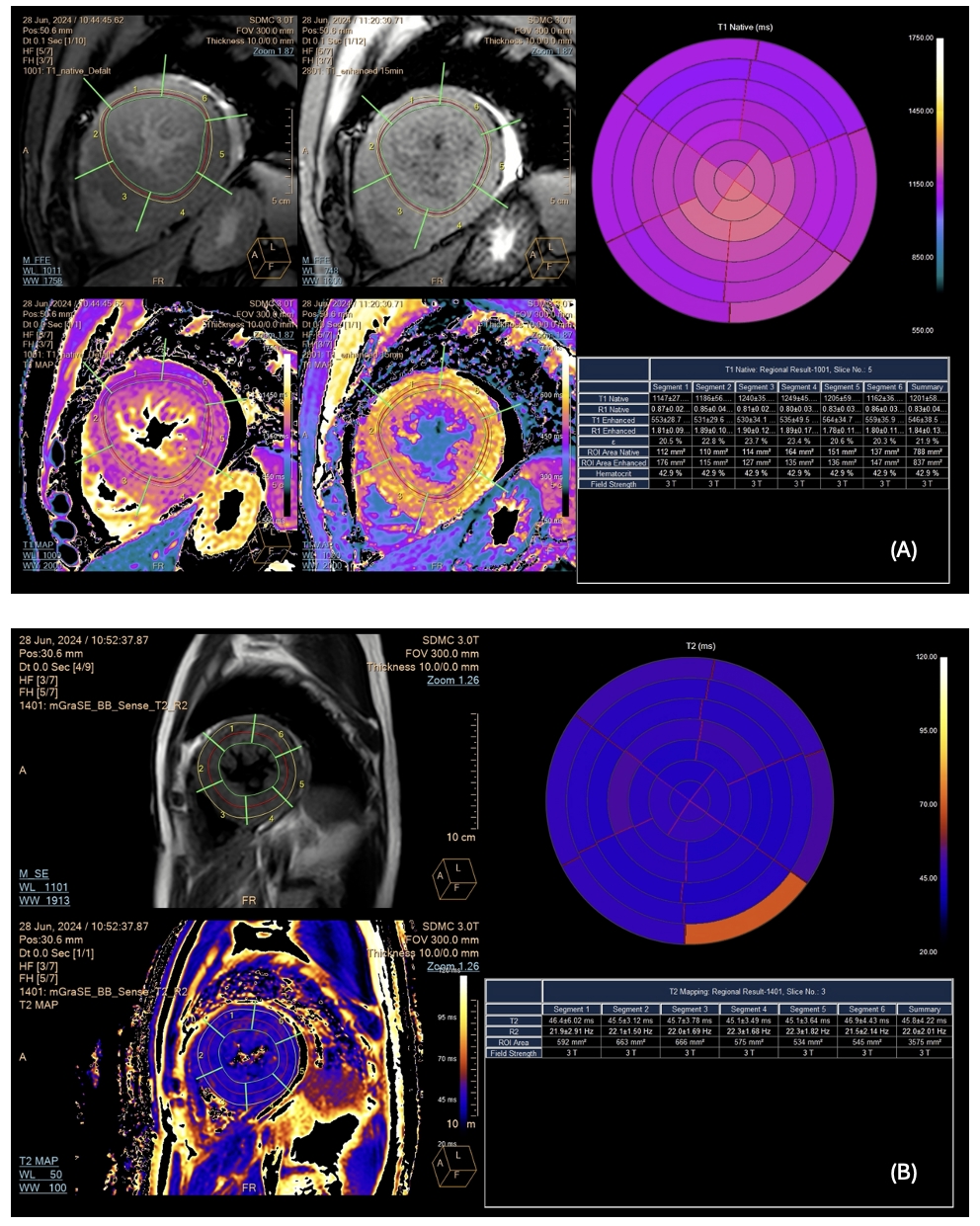
CMR confirmed increased LV wall thickness (maximum 22.68 mm at the inferoseptal segment) and identified an accessory papillary muscle with papillary muscle hypertrophy. The apical insertion of the papillary muscle was observed, contributing to chordal systolic anterior motion (SAM) (Figure2). Delayed enhancement showed no hyperenhancement of both ventricles. The tissue characterization showed normal native T1 values (global native T1 1201 ± 58 ms, site normal reference 1231 ± 23.6 ms at 3 Tesla) and no increase in T2 relaxation time (global native T2 45.8 ± 4.2 ms) from the mapping sequences (Figure 3).
Learning Points from this Case:
The coexistence of Andersen-Fabry disease (AFD) and hypertrophic cardiomyopathy (HCM) presents significant diagnostic and management challenges, as both conditions can independently cause LV hypertrophy. In this case, the presence of typical HCM features, such as abnormal papillary muscle insertion, papillary muscle hypertrophy, and SAM was observed, along with the presence of pathogenic mutation in ALPK3 which associated with an autosomal dominant adult-onset HCM. Additionally, tissue characterization did not reveal the usual AFD-associated findings, such as decreased native T1 values. This lack of typical AFD markers might be attributed to a pseudonormalization effect caused by fibrosis or the combined impact of HCM. This case emphasizes the importance of comprehensive evaluation in patients with AFD to accurately identify and manage the potential interactions with HCM.
The echocardiography demonstrated increased LV wall thickness (1A-1B). The pulse waved (PW) doppler showed a mid-cavitary gradient of 20 mmHg at rest, which increasing to 60 mmHg during the Valsalva maneuver (1C-1D)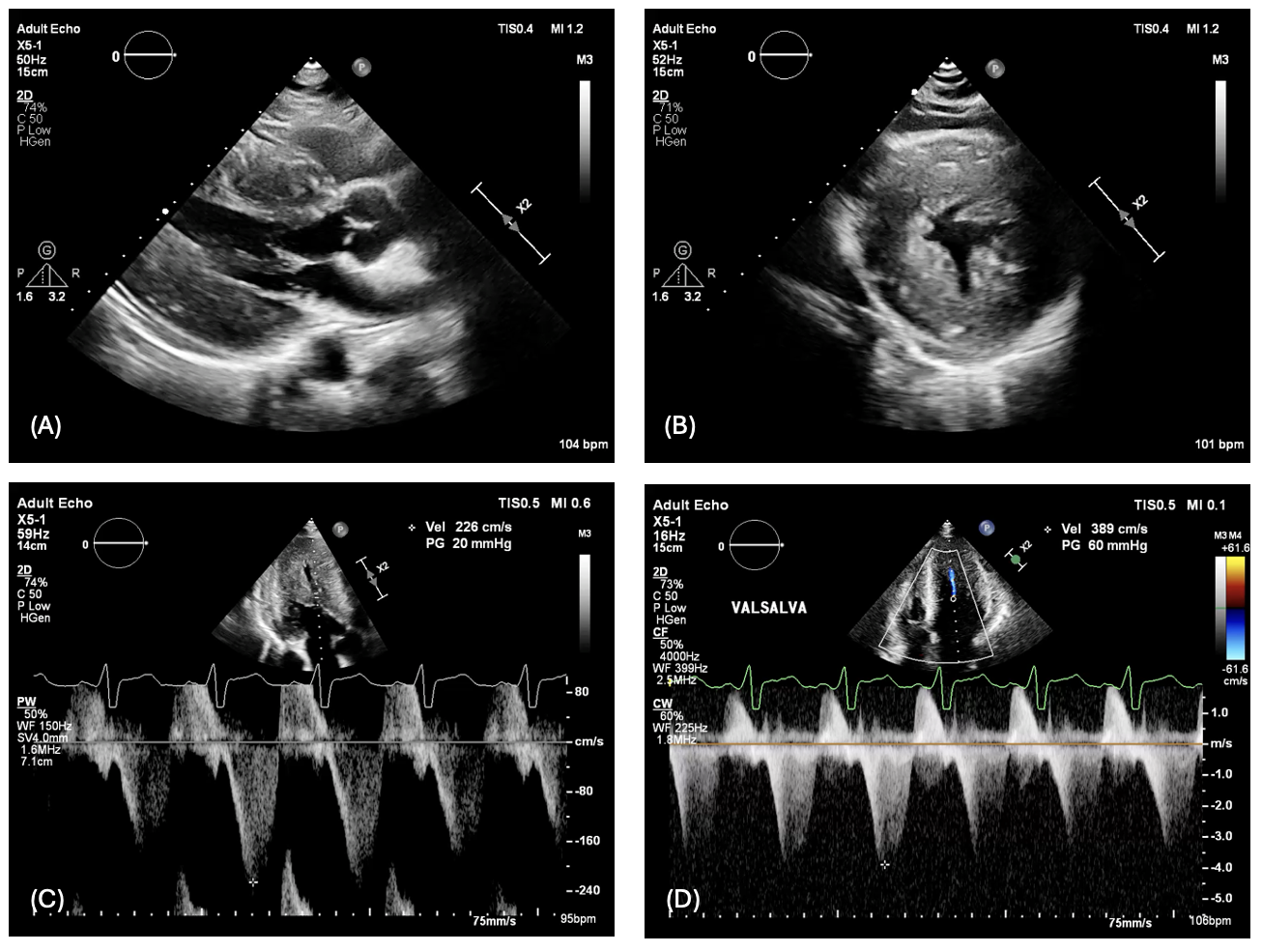
CMR demonstrated increased LV wall thickness (2A) and identified an accessory papillary muscle (star) with papillary muscle hypertrophy. The apical insertion of the papillary muscle was observed from 4 chamber view (2B) which contributing to chordal systolic anterior motion (2C)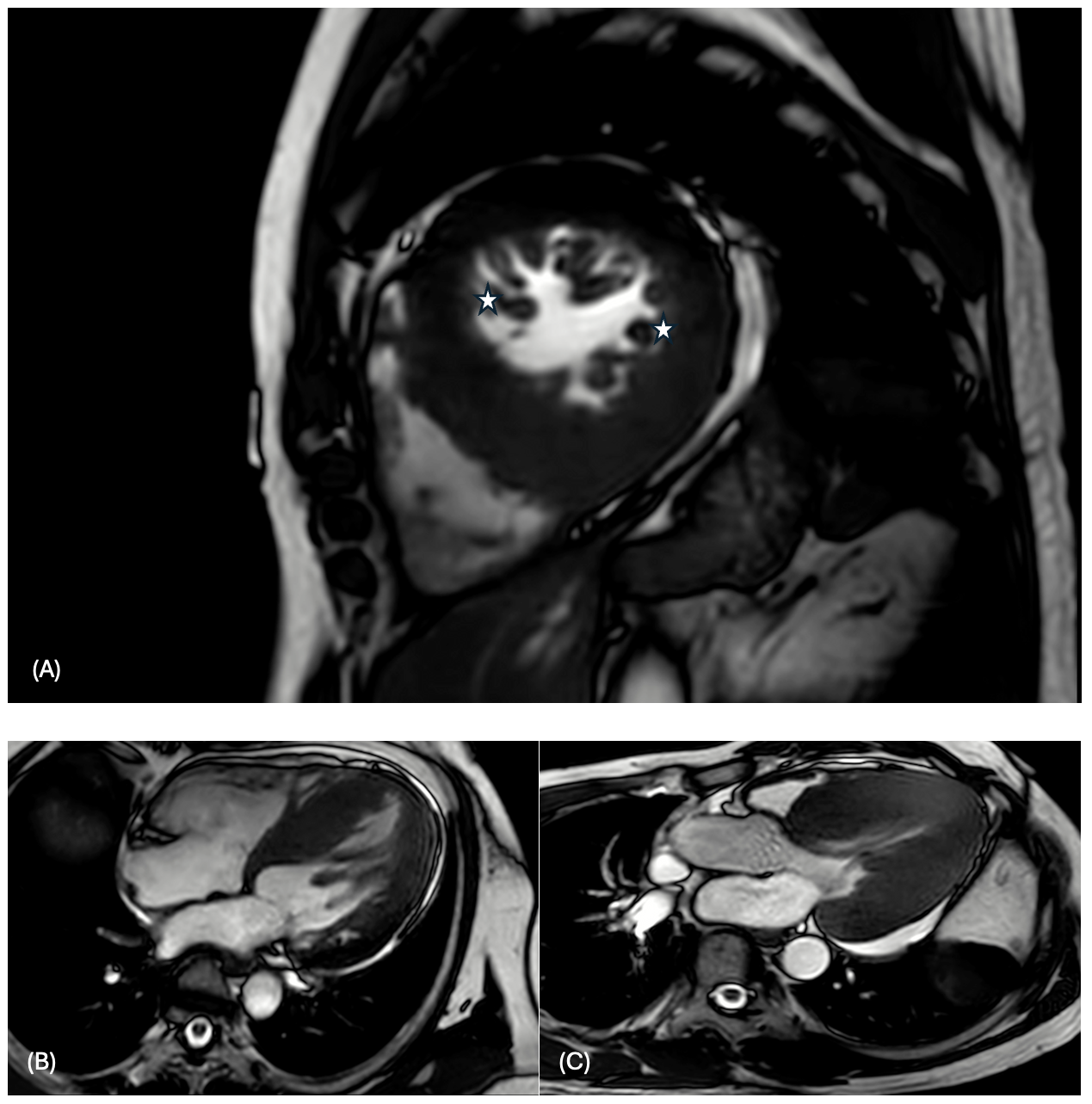
The tissue characterization showed normal global native T1 values (global native T1 1201 ± 58 ms) from T1 mapping sequence (3A) and no increase in T2 relaxation time (global native T2 45.8 ± 4.2 ms) from T2 mapping sequence (3B).