Rapid Fire Abstracts
Automating Real-Time Flow Quantification and Alias Detection with Deep Learning (RF_TH_168)
- HC
Hoi Ching Cheung, MD, BSc
Honorary Clinical Research Fellow
National Heart and Lung Institute, Imperial College London, United Kingdom - MM
Michalis Michaelides, BSc
Undergraduate
Imperial College London, United Kingdom - MM
Muhammad Mohsin, BSc
Undergraduate
Imperial College London, United Kingdom - AS
Ahmed Salih, BSc
Undergraduate
Imperial College London, United Kingdom - SZ
Sameer Zaman, MD, PhD
Honorary Clinical Lecturer
National Heart and Lung Institute, Imperial College London, United Kingdom - KV
Kavitha Vimalesvaran, MD, PhD
Clinical Research Fellow
National Heart and Lung Institute, Imperial College London, United Kingdom 
Graham Cole, MD, PhD
Consultant Cardiologist
Imperial College London, United Kingdom- AS
Alexander Stevenson, MD, BA
Specialist registrar in Cardiology
Chelsea and Westminster Hospital NHS Foundation Trust, United Kingdom - JH
James Howard, MD, PhD
Senior Lecturer and Honorary Consultant Cardiologist
National Heart and Lung Institute, Imperial College London, United Kingdom
Presenting Author(s)
Co-Author(s)
Primary Author(s)
Background:
Accurate measurement of cardiac blood flow using phase-contrast cardiac magnetic resonance imaging (MRI) is essential for the diagnosis, monitoring, and management of cardiac diseases. Traditionally, this requires manual image inspection during acquisition and dynamic adjustment of parameters such as encoding velocity to prevent aliasing artefacts, which can compromise diagnostic accuracy. After images are acquired, vessels must be manually contoured frame-by-frame, in order to derive flow parameters over the cardiac cycle. Our study aimed to develop an artificial intelligence (AI) model to automate these laborious processes, by predicting contours of the great vessels of the heart to achieve real-time detection of aliasing artefact and measurement of blood flow. We aimed to deploy this AI on the MRI scanners at our hospital, allowing automated quality control and identification of pathology.
Methods: A two-dimensional Efficient-U-Net was designed and trained for the segmentation of the aorta, the main pulmonary artery, the left pulmonary artery, and the right pulmonary artery. The dataset was split at the patient level into training, validation, and testing subsets in a 3:1:1 ratio. Vessel contours were manually traced by four operators and subsequently reviewed by a Level 3 CMR expert. Additionally, the cines were annotated to indicate the presence or absence of aliasing. The model’s performance was evaluated using Intersection over Union (IoU) for segmentation accuracy and the Area Under the Receiver Operating Characteristic (AUROC) curve for alias detection. The AI model was implemented on Siemens 1.5T Aera scanner, integrated with the Siemens Framework for Image Reconstruction Environments (FIRE).
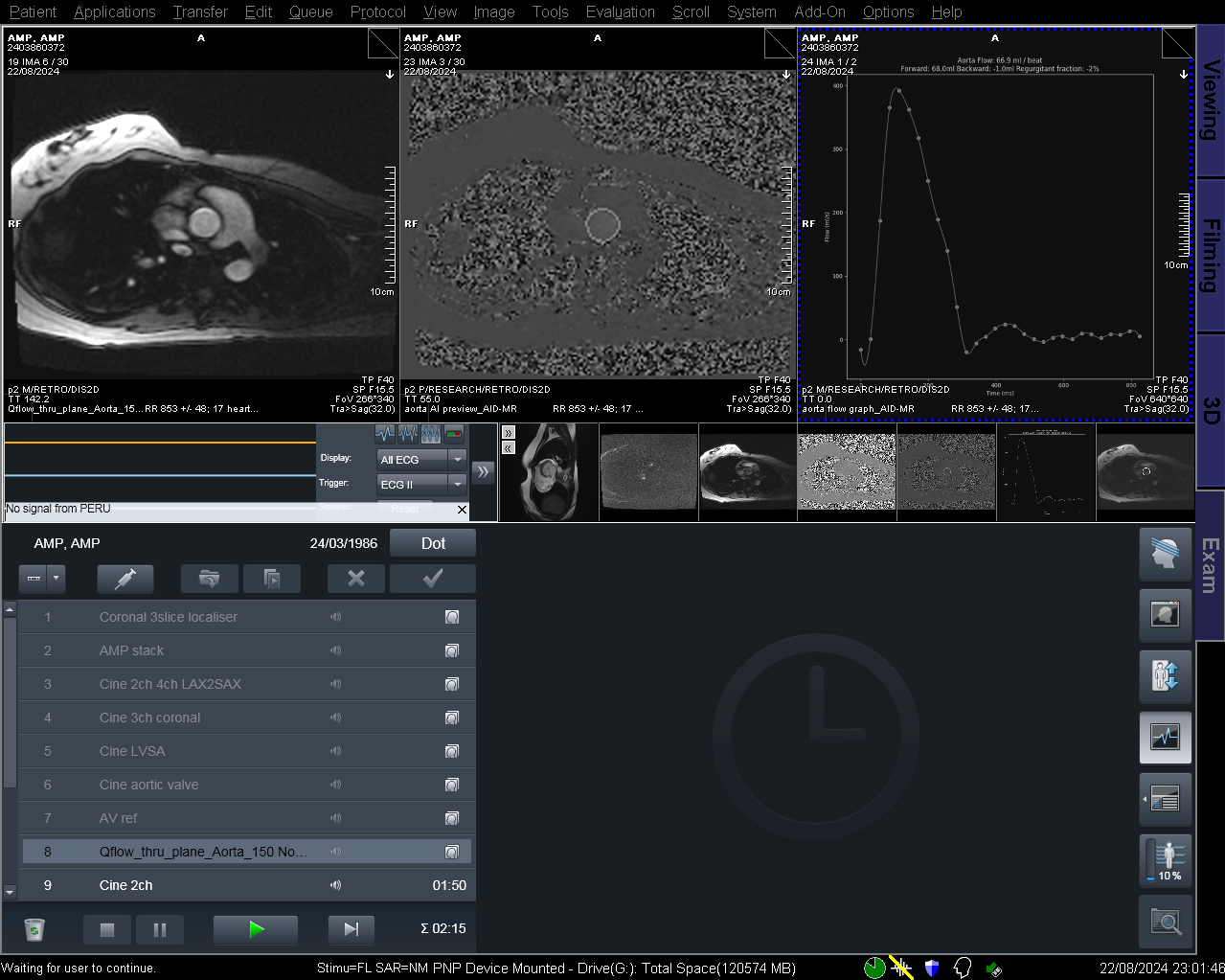
Results: A total of 387 series (11675 frames) were included (Table 1). 239, 82, and 66 series were assigned to the training, validation and testing datasets, respectively. The model demonstrated strong agreement with expert annotations, achieving an overall IoU of 0.92, with class-wise IoU of 0.87 (aorta), 0.83 (main pulmonary artery (PA)), 0.82 (left PA) and 0.77 (right PA). Using the AI predicted masks, the model was able to identify aliasing with high efficacy (AUROC 0.98 (95% confidence interval 0.94-1.00), accuracy 94%, sensitivity 100%, specificity 86%). Figure 1 shows the acquired sequence, automated segmentation, and flow curve within the scanning interface.
Conclusion: Accurate automated flow contouring of the aorta, main, left, and right pulmonary arteries is feasible with AI. The derived segmentation maps enable the real-time detection of aliasing artefacts, improving diagnostic accuracy and workflow efficiency. This solution has been successfully implemented on scanners at our hospital.
Real-time inference of the AI model on a Siemens 1.5 T Aera scanner. From left to right, the top panel shows the acquired sequence, the AI predicted tracing of the vessel of interest and the derived flow values respectively.

