ISMRM - SCMR Workshop
Power Pitch 4: Repeatability and Reproducibility of Accelerated Free Breathing Motion-Compensated Spin-Echo Cardiac Diffusion Tensor Imaging
- TW
Tess E. Wallace, PhD
Senior Clinical MR Research Scientist
Siemens Medical Solutions USA, Inc. - TW
Tess E. Wallace, PhD
Senior Clinical MR Research Scientist
Siemens Medical Solutions USA, Inc. - KM
Kevin Moulin, PhD
Research Scientist
Boston Children's Hospital - JR
Jennifer Rodriguez
Clinical Trials Specialist
Beth Israel Deaconess Medical Center - JS
Jordan A. Street, BA
Clinical Research Coordinator
Beth Israel Deaconess Medical Center - SJ
Scott Johnson
MRI Tech
Beth Israel Deaconess Medical Center - PP
Patrick Pierce, BSc
MRI Technologist
Beth Israel Deaconess Medical Center .jpg)
Magalie Viallon, PhD
Researcher
Univ Lyon, UJM-Saint-Etienne, INSA, CNRS UMR 5520, INSERM U1206, CREATIS, France.jpg)
Andy Powell, MD
Physician
Boston Children's Hospital
Boston Children's Hospital- RN
Reza Nezafat, PhD
Professor
Harvard Medical School
Presenting Author(s)
Primary Author(s)
Co-Author(s)
Diffusion tensor CMR (DT-CMR) is most often applied at 3T to improve SNR and minimise scan times but this limits clinical applicability which would be improved through application at the more available field strength of 1.5T. Additionally, in vivo DT-CMR challenges patients by requiring multiple long breath-holds (BH), motivating the development of free-breathing (FB) approaches 1-4 including at 1.5T using diastolic FB spin echo without M1M2 cardiac motion compensation5. We set out to evaluate the feasibility of acquiring systolic DT CMR at 1.5T using a FB navigator guided slice-tracked M1M2-SE and compared its repeatability and bias to the BH-mode.
Methods:
We recruited 12 healthy volunteers (5 females; age range 18-60 years) at 1.5T ( Avanto FIT, Siemens, Erlangen, Germany, 45 mT/m, 200 T/m/s). A mid short axis cine acquisition was used to find the LV end-systolic timing for subject-specific calculation of trigger delay for M1M2-SE6. The M1M2 zone-selective7 SE sequence parameters were: FOV 360x135mm, TE 70ms, MDDW peak 36.6mT/m per axis, acquired resolution 2.8x2.8x10mm, parallel imaging factor 2, no partial Fourier8.
For BH SE DT-CMR, we acquired alternate cycles for T1 recovery, 17RR BHs, 8 BH averages at b=350 s/mm2 (“b350”) and 2 BHs at b50. For FB DT-CMR, a diaphragm navigator slice tracked (fixed factor 0.6) sequence 10 acquired 16 averages at b350 and 4 at b50, imaging in every cycle. The BH and FB acquisitions were each run twice for intra-sessional reproducibility and to assess any bias.
Custom MATLAB software9 obtained pixelwise mean diffusivity (MD), fractional anisotropy (FA) and helix angle (HA) maps. Each HA map was divided into 12 angular segments in which the expected transmural HA variation was scored (0-12). The averages in the corresponding MD and FA map segments were taken for the results. During analysis, images with large respiratory shifts or other reasons for myocardial signal disruption were manually rejected.
Results:
Two subjects were unused (HA map score < 6). In the 10 included, mean cardiac cycle was 895ms (650-1200ms), weight range 58-90 kg. Table 1 summarises the plotted results (Fig 1), with statistical tests showing no significant differences between BH and FB. The average acquisition time for BH SE DTI (including BH instruction and rest times between each 17RR BH) was 6’6”, and 3’10” for FB. Fig 2 shows examples of diffusion parameter maps.
Discussion
We show for the first time that at 1.5T, a free-breathing navigator guided slice-tracked M1M2-SE had comparable scatter to breath-held M1M2SE without causing bias. The MD values are within systolic ranges8 under similar acquisition conditions, however the FA values are overestimated, possibly due to the low (b350) diffusion sensitivity used for these initial experiments on a busy clinical scanner. The SE DTI EPI could be adapted to improve SNR at 1.5T, trading against extended TE, possibly speeding up FB acquisition for multislice scanning. The reported robustness during contraction into systole6 and sufficient diffusion sensitivity may demand higher gradient performance than used here8. Future scans mandate a “timing scout” to improve reliability.
Conclusion:
Free-breathing systolic navigator guided slice-tracked M1M2 compensated 1.5T spin echo (M1M2-SE) DT-CMR did not cause bias or lose precision compared to BH. This work offers wider clinical utility for the systolic assessment of cardiac microstructure, by working at 1.5T at standard gradient-performance and by free-breathing mitigating the need for multiple long BHs.
Table 1: Results (n=10) with statistical tests.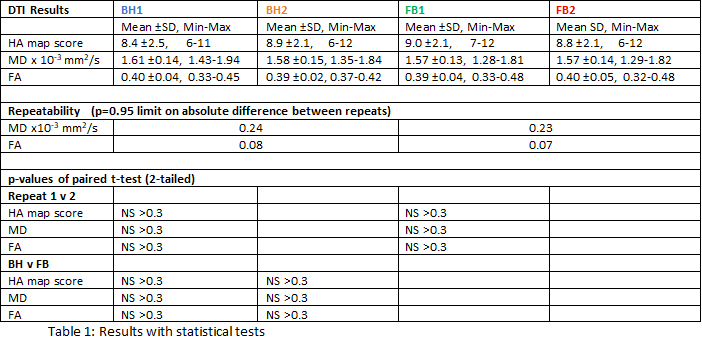
Figure 1: Graphs comparing the mean effect and scatter of mid-ventricular mean diffusivity and fractional anisotropy assessed in 10 healthy volunteers acquired using breath-hold (BH) M1M2SE and free-breathing (FB) M1M2SE.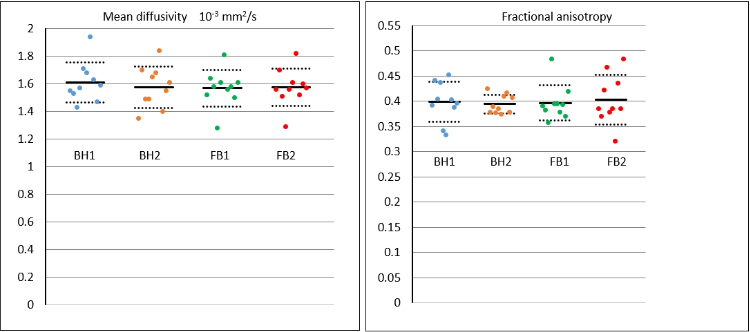
Figure 2. a) Subject with HA map score 12 for BH and FB. b) Subject with HA map scores 6 for BH and 7 for FB, where only the anterior and antero-septal values were averaged for MD and FA. No average improvement by FB is implied. .png)
Figure 1. Example trace diffusion-weighted images (DWI) and derived parametric DTI maps of mean diffusivity (MD), fractional anisotropy (FA), helix angle (HA) and absolute secondary eigenvector angle (E2A) in A) the two sessions of four repeated scans in a representative healthy subject and B) the two repeated scans in a patient with hypertrophic cardiomyopathy (HCM)..jpg)
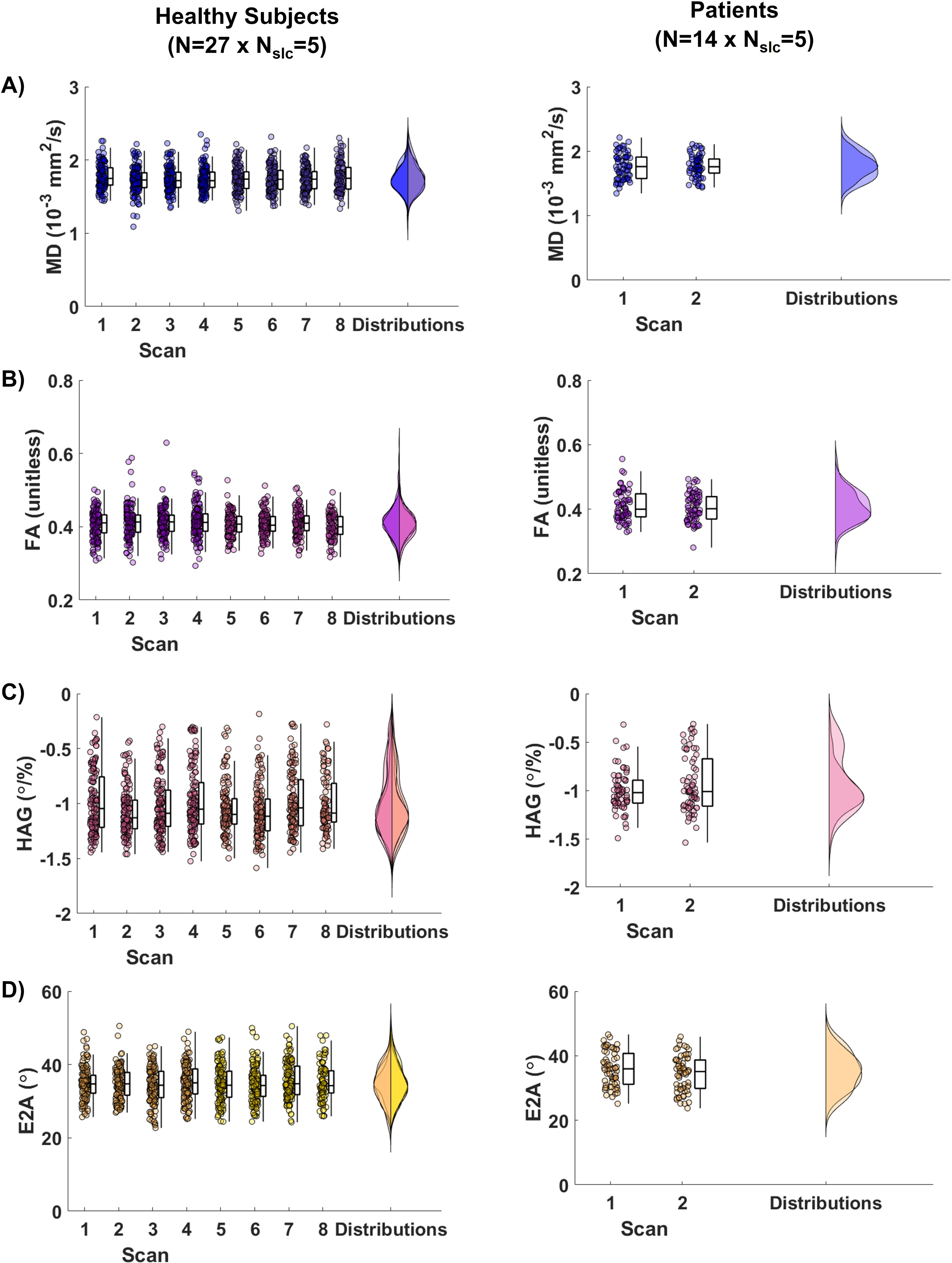
Figure 2. Distributions of (A) mean diffusivity (MD), (B) fractional anisotropy (FA), (C) helix angle gradient (HAG), and (D) absolute secondary eigenvector angle (E2A) for all scans and sessions in healthy subjects (N=27) and in patients (N=14). Each data point represents a slice in each scan.